Oligopeptides analysis in spiderhawk's venom (Pepsis decorata Perty, 1833, Hymenoptera: Pompilidae)
IF 1.7
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Wasps have been neglected in toxinological studies, even with their diversity of species, when compared to other groups of venomous animals such as snakes, scorpions, and spiders. Solitary wasps, such as Pepsis decorata, are known for their mechanism of total or temporary paralysis of the host. In addition, their venoms are considered sources for studies of small peptides, bioactive peptides with neural and antimicrobial activities. In this work, some oligopeptides were analyzed by de novo sequencing identifying 39 oligopeptide sequences. Some sequences were similar to proctolin, a bradykinin-potentiating peptide, and poneritoxin, one bradykinin-related peptide. As proctolin-like peptides were the major constituent in distinct experimental conditions, it was selected for further in silico studies in order to understand its possible importance as a constituent of wasp venom and whether these peptides could be of biotechnological importance. We investigate its binding mode comparing with proctolin and we further analyzed the importance of the tyrosine-leucine-glutamic acid (YLE) tripeptide-motif conservation. This experimental, an in silico approach, increased the range of compounds identified in peptide analyses proving good characterization of little-known peptidic compounds.
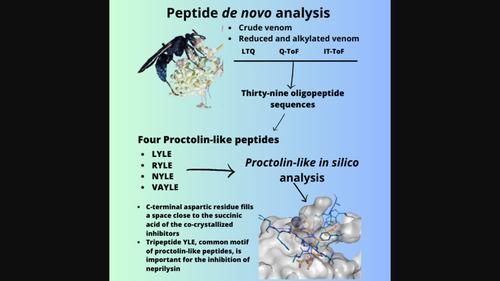
蛛鹰(Pepsis decorata Perty,1833,膜翅目:鲳科)毒液中的寡肽分析
与蛇、蝎子和蜘蛛等其他有毒动物相比,黄蜂的种类繁多,但在毒素学研究中一直被忽视。独居黄蜂(如 Pepsis decorata)因其完全或暂时麻痹宿主的机制而闻名。此外,它们的毒液也被认为是研究小肽、具有神经和抗菌活性的生物活性肽的来源。在这项工作中,通过重新测序分析了一些寡肽,确定了 39 个寡肽序列。其中一些序列与缓激肽刺激肽 Proctolin 和缓激肽相关肽 poneritoxin 相似。由于在不同的实验条件下,类似于proctolin的肽是主要成分,因此我们选择了它进行进一步的硅学研究,以了解它作为黄蜂毒液成分的可能重要性,以及这些肽是否具有生物技术的重要性。我们研究了它与前列腺素的结合模式,并进一步分析了酪氨酸-亮氨酸-谷氨酸(YLE)三肽-motif 保护的重要性。这项实验采用了硅学方法,增加了在肽分析中发现的化合物范围,证明对鲜为人知的多肽化合物进行了很好的表征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


