Generation of Functional and Mature Sympathetic Neurons from Human Pluripotent Stem Cells via a Neuroepithelial Route
Abstract
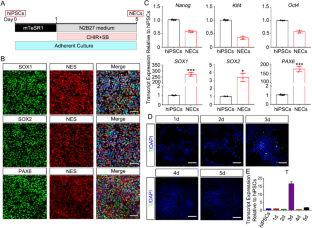
The sympathetic nervous system (SNS) is a crucial branch of the autonomic nervous system (ANS) that is responsible for regulating visceral function and various physiological processes. Dysfunction of the SNS can lead to various diseases, such as hypertension and metabolic disorders. However, obtaining sympathetic neurons from human tissues for research is challenging. The current research aimed at recapitulating the process of human sympathetic neuron development and achieved the successful establishment of a stepwise, highly efficient in vitro differentiation protocol. This protocol facilitated the generation of functional and mature sympathetic neurons from human pluripotent stem cells (hPSCs) using a chemical-defined induction medium. Initially, each differentiation stage was refined to derive sympathoadrenal progenitors (SAPs) from hPSCs through neural epithelial cells (NECs) and trunk neural crest stem cells (NCSCs). hPSC-derived SAPs could be expanded in vitro for at least 12 passages while maintaining the expression of SAP-specific transcription factors and neuronal differentiation potency. SAPs readily generated functional sympathetic neurons (SymNs) when cultured in the neuronal maturation medium for 3–4 weeks. These SymNs expressed sympathetic markers, exhibited electrophysiological properties, and secreted sympathetic neurotransmitters. More importantly, we further demonstrated that hPSC-derived SymNs can efficiently regulate the adipogenesis of human adipose–derived stem cells (ADSCs) and lipid metabolism in vitro. In conclusion, our study provided a simple and robust protocol for generating functional sympathetic neurons from hPSCs, which may be an invaluable tool in unraveling the mechanisms of SNS-related diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


