CD200Rhigh neutrophils with dysfunctional autophagy establish systemic immunosuppression by increasing regulatory T cells
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
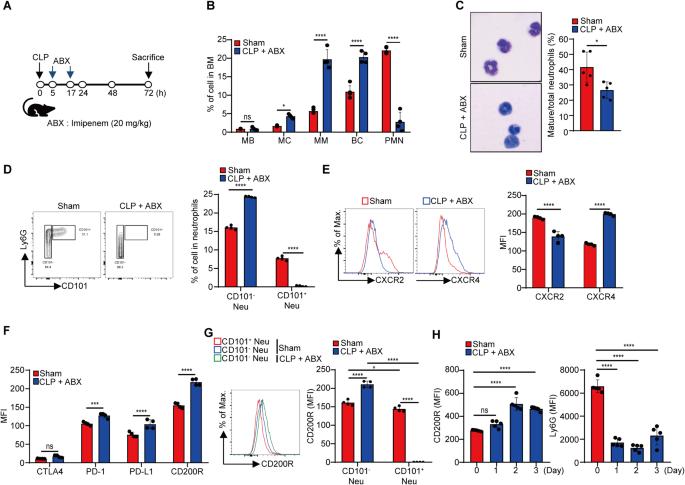
Distinct neutrophil populations arise during certain pathological conditions. The generation of dysfunctional neutrophils during sepsis and their contribution to septicemia-related systemic immune suppression remain unclear. In this study, using an experimental sepsis model that features immunosuppression, we identified a novel population of pathogenic CD200Rhigh neutrophils that are generated during the initial stages of sepsis and contribute to systemic immune suppression by enhancing regulatory T (Treg) cells. Compared to their CD200Rlow counterparts, sepsis-generated CD200Rhigh neutrophils exhibit impaired autophagy and dysfunction, with reduced chemotactic migration, superoxide anion production, and TNF-α production. Increased soluble CD200 blocks autophagy and neutrophil maturation in the bone marrow during experimental sepsis, and recombinant CD200 treatment in vitro can induce neutrophil dysfunction similar to that observed in CD200Rhigh neutrophils. The administration of an α-CD200R antibody effectively reversed neutrophil dysfunction by enhancing autophagy and protecting against a secondary infection challenge, leading to increased survival. Transcriptome analysis revealed that CD200Rhigh neutrophils expressed high levels of Igf1, which elicits the generation of Treg cells, while the administration of an α-CD200R antibody inhibited Treg cell generation in a secondary infection model. Taken together, our findings revealed a novel CD200Rhigh neutrophil population that mediates the pathogenesis of sepsis-induced systemic immunosuppression by generating Treg cells.
自噬功能失调的 CD200R 高中性粒细胞会通过增加调节性 T 细胞来建立全身免疫抑制。
在某些病理条件下会出现不同的中性粒细胞群。脓毒症期间功能失调中性粒细胞的产生及其对脓毒症相关全身免疫抑制的贡献仍不清楚。在这项研究中,我们利用一种以免疫抑制为特征的实验性脓毒症模型,发现了一种新的致病性 CD200Rhigh 中性粒细胞群,它们在脓毒症初期产生,并通过增强调节性 T(Treg)细胞来促进全身免疫抑制。与 CD200R 低的中性粒细胞相比,脓毒症产生的 CD200R 高的中性粒细胞表现出自噬受损和功能障碍,趋化性迁移、超氧阴离子生成和 TNF-α 生成减少。在实验性脓毒症期间,可溶性 CD200 的增加会阻碍骨髓中的自噬和中性粒细胞成熟,体外重组 CD200 可诱导中性粒细胞功能障碍,与 CD200Rhigh 中性粒细胞中观察到的功能障碍相似。服用α-CD200R抗体可通过增强自噬和抵御二次感染挑战来有效逆转中性粒细胞功能障碍,从而提高存活率。转录组分析表明,CD200R高的中性粒细胞表达高水平的Igf1,而Igf1能诱导Treg细胞的生成,而服用α-CD200R抗体能抑制二次感染模型中Treg细胞的生成。总之,我们的研究结果揭示了一种新型的高CD200R中性粒细胞群,它通过生成Treg细胞介导脓毒症诱导的全身免疫抑制的发病机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


