Detailed DNA methylation characterisation of phyllodes tumours identifies a signature of malignancy and distinguishes phyllodes from metaplastic breast carcinoma
IF 5.6
2区 医学
Q1 ONCOLOGY
Braydon Meyer, Clare Stirzaker, Sonny Ramkomuth, Kate Harvey, Belinda Chan, Cheok Soon Lee, Rooshdiya Karim, Niantao Deng, Kelly A Avery-Kiejda, Rodney J Scott, Sunil Lakhani, Stephen Fox, Elizabeth Robbins, Joo-Shik Shin, Jane Beith, Anthony Gill, Loretta Sioson, Charles Chan, Mrudula Krishnaswamy, Caroline Cooper, Sanjay Warrier, Cindy Mak, John EJ Rasko, Charles G Bailey, Alexander Swarbrick, Susan J Clark, Sandra O'Toole, Ruth Pidsley
下载PDF
{"title":"Detailed DNA methylation characterisation of phyllodes tumours identifies a signature of malignancy and distinguishes phyllodes from metaplastic breast carcinoma","authors":"Braydon Meyer, Clare Stirzaker, Sonny Ramkomuth, Kate Harvey, Belinda Chan, Cheok Soon Lee, Rooshdiya Karim, Niantao Deng, Kelly A Avery-Kiejda, Rodney J Scott, Sunil Lakhani, Stephen Fox, Elizabeth Robbins, Joo-Shik Shin, Jane Beith, Anthony Gill, Loretta Sioson, Charles Chan, Mrudula Krishnaswamy, Caroline Cooper, Sanjay Warrier, Cindy Mak, John EJ Rasko, Charles G Bailey, Alexander Swarbrick, Susan J Clark, Sandra O'Toole, Ruth Pidsley","doi":"10.1002/path.6250","DOIUrl":null,"url":null,"abstract":"<p>Phyllodes tumours (PTs) are rare fibroepithelial lesions of the breast that are classified as benign, borderline, or malignant. As little is known about the molecular underpinnings of PTs, current diagnosis relies on histological examination. However, accurate classification is often difficult, particularly for distinguishing borderline from malignant PTs. Furthermore, PTs can be misdiagnosed as other tumour types with shared histological features, such as fibroadenoma and metaplastic breast cancers. As DNA methylation is a recognised hallmark of many cancers, we hypothesised that DNA methylation could provide novel biomarkers for diagnosis and tumour stratification in PTs, whilst also allowing insight into the molecular aetiology of this otherwise understudied tumour. We generated whole-genome methylation data using the Illumina EPIC microarray in a novel PT cohort (<i>n</i> = 33) and curated methylation microarray data from published datasets including PTs and other potentially histopathologically similar tumours (total <i>n</i> = 817 samples). Analyses revealed that PTs have a unique methylome compared to normal breast tissue and to potentially histopathologically similar tumours (metaplastic breast cancer, fibroadenoma and sarcomas), with PT-specific methylation changes enriched in gene sets involved in KRAS signalling and epithelial-mesenchymal transition. Next, we identified 53 differentially methylated regions (DMRs) (false discovery rate < 0.05) that specifically delineated malignant from non-malignant PTs. The top DMR in both discovery and validation cohorts was hypermethylation at the <i>HSD17B8</i> CpG island promoter. Matched PT single-cell expression data showed that <i>HSD17B8</i> had minimal expression in fibroblast (putative tumour) cells. Finally, we created a methylation classifier to distinguish PTs from metaplastic breast cancer samples, where we revealed a likely misdiagnosis for two TCGA metaplastic breast cancer samples. In conclusion, DNA methylation alterations are associated with PT histopathology and hold the potential to improve our understanding of PT molecular aetiology, diagnostics, and risk stratification. © 2024 The Authors. <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"262 4","pages":"480-494"},"PeriodicalIF":5.6000,"publicationDate":"2024-02-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6250","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6250","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
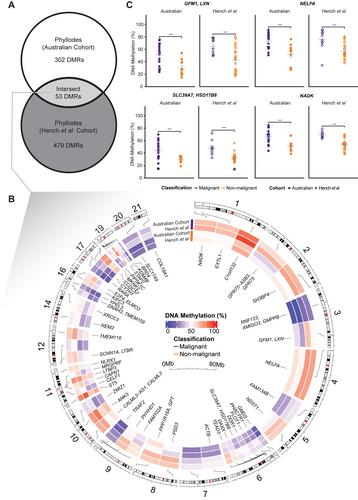
Phyllodes tumours (PTs) are rare fibroepithelial lesions of the breast that are classified as benign, borderline, or malignant. As little is known about the molecular underpinnings of PTs, current diagnosis relies on histological examination. However, accurate classification is often difficult, particularly for distinguishing borderline from malignant PTs. Furthermore, PTs can be misdiagnosed as other tumour types with shared histological features, such as fibroadenoma and metaplastic breast cancers. As DNA methylation is a recognised hallmark of many cancers, we hypothesised that DNA methylation could provide novel biomarkers for diagnosis and tumour stratification in PTs, whilst also allowing insight into the molecular aetiology of this otherwise understudied tumour. We generated whole-genome methylation data using the Illumina EPIC microarray in a novel PT cohort (n = 33) and curated methylation microarray data from published datasets including PTs and other potentially histopathologically similar tumours (total n = 817 samples). Analyses revealed that PTs have a unique methylome compared to normal breast tissue and to potentially histopathologically similar tumours (metaplastic breast cancer, fibroadenoma and sarcomas), with PT-specific methylation changes enriched in gene sets involved in KRAS signalling and epithelial-mesenchymal transition. Next, we identified 53 differentially methylated regions (DMRs) (false discovery rate < 0.05) that specifically delineated malignant from non-malignant PTs. The top DMR in both discovery and validation cohorts was hypermethylation at the HSD17B8 CpG island promoter. Matched PT single-cell expression data showed that HSD17B8 had minimal expression in fibroblast (putative tumour) cells. Finally, we created a methylation classifier to distinguish PTs from metaplastic breast cancer samples, where we revealed a likely misdiagnosis for two TCGA metaplastic breast cancer samples. In conclusion, DNA methylation alterations are associated with PT histopathology and hold the potential to improve our understanding of PT molecular aetiology, diagnostics, and risk stratification. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
对鳞状细胞瘤进行详细的 DNA 甲基化分析,确定了恶性肿瘤的特征,并将鳞状细胞瘤与移行细胞乳腺癌区分开来。
鳞状上皮细胞瘤(PTs)是一种罕见的乳腺纤维上皮病变,可分为良性、边缘性或恶性。由于对PT的分子基础知之甚少,目前的诊断主要依靠组织学检查。然而,准确的分类往往很困难,尤其是在区分边缘型和恶性 PT 时。此外,PT 还可能被误诊为具有共同组织学特征的其他肿瘤类型,如纤维腺瘤和变性乳腺癌。由于DNA甲基化是许多癌症的公认标志,我们假设DNA甲基化能为PT的诊断和肿瘤分层提供新的生物标记物,同时也能深入了解这种未被充分研究的肿瘤的分子病因。我们使用 Illumina EPIC 微阵列在一个新型 PT 队列(n = 33)中生成了全基因组甲基化数据,并从已发表的数据集(包括 PT 和其他可能组织病理学相似的肿瘤)中整理了甲基化微阵列数据(共 n = 817 个样本)。分析表明,与正常乳腺组织和组织病理学上可能相似的肿瘤(移行细胞乳腺癌、纤维腺瘤和肉瘤)相比,PT 具有独特的甲基化组,PT 特异性甲基化变化富集在参与 KRAS 信号传导和上皮-间质转化的基因集中。接下来,我们确定了 53 个差异甲基化区域(DMR)(假发现率
本文章由计算机程序翻译,如有差异,请以英文原文为准。
来源期刊
期刊介绍:
The Journal of Pathology aims to serve as a translational bridge between basic biomedical science and clinical medicine with particular emphasis on, but not restricted to, tissue based studies. The main interests of the Journal lie in publishing studies that further our understanding the pathophysiological and pathogenetic mechanisms of human disease.
The Journal of Pathology welcomes investigative studies on human tissues, in vitro and in vivo experimental studies, and investigations based on animal models with a clear relevance to human disease, including transgenic systems.
As well as original research papers, the Journal seeks to provide rapid publication in a variety of other formats, including editorials, review articles, commentaries and perspectives and other features, both contributed and solicited.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


