Pd-Catalyzed Carbothiolation Using Thioesters with Forming a Quaternary Carbon
引用次数: 0
Abstract
The Pd-catalyzed carbothiolation using thioesters with forming a quaternary carbon is described. The carbothiolation using thioesters was problematic due to a direct coupling side reaction that produced a sulfide, but this side reaction was successfully suppressed by selecting the thioester and reaction conditions. When chroman and coumaran derivatives were obtained in this reaction, the reactions with S-phenyl 4-methoxybenzothioate, Pd(PPh3)4, and Cs2CO3 at 100 °C in toluene afforded the desired products in good yields (77-92%). The carbothiolation reaction also proceeded using the ester of alkane thiol with higher yields (56-93%) when compared with the previously reported carbothiolation using TIPS thioethers (12-63%). The developed Pd-catalyzed carbothiolation is applicable for the preparation of a wide range of products including a tetralin derivative and an indoline derivative, too. The Pd-catalyzed carbothiolation using thioesters was found to be comparable to previously reported carbothiolation using TIPS thioethers in terms of yield and substrate scope, and to be a superior alternative owing to the stability and lower cost of thioesters.
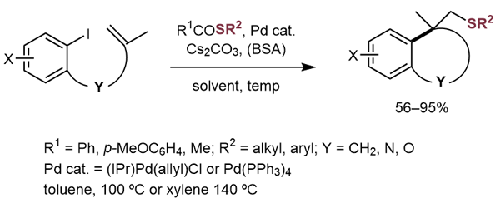
Pd 催化硫代酯硫代碳化反应,形成季碳
本文介绍了利用硫代酯进行 Pd 催化硫代碳化反应并形成季碳的方法。由于直接偶联副反应会产生硫化物,使用硫代酯进行硫代碳化反应存在问题,但通过选择硫代酯和反应条件,成功地抑制了这一副反应。当在该反应中得到色满和香豆素衍生物时,在 100 °C 的甲苯中与 4-甲氧基硫代苯甲酸 S-苯基、Pd(PPh3)4 和 Cs2CO3 反应,可以得到所需的产物,收率很高(77-92%)。与之前报道的使用 TIPS 硫醚进行硫代碳化反应(12-63%)相比,使用烷硫醇酯进行硫代碳化反应的产率更高(56-93%)。所开发的钯催化硫代碳化法适用于制备多种产品,包括四氢萘衍生物和吲哚啉衍生物。研究发现,在产率和底物范围方面,使用硫代酯的 Pd 催化硫代碳化法与之前报道的使用 TIPS 硫代醚的硫代碳化法相当,而且由于硫代酯的稳定性和较低成本,Pd 催化硫代碳化法是一种更优越的替代方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


