Developmental morphogens direct human induced pluripotent stem cells toward an annulus fibrosus-like cell phenotype
Abstract
Introduction
Therapeutic interventions for intervertebral disc herniation remain scarce due to the inability of endogenous annulus fibrosus (AF) cells to respond to injury and drive tissue regeneration. Unlike other orthopedic tissues, such as cartilage, delivery of exogenous cells to the site of annular injury remains underdeveloped, largely due to a lack of an ideal cell source and the invasive nature of cell isolation. Human induced pluripotent stem cells (iPSCs) can be differentiated to specific cell fates using biochemical factors and are, therefore, an invaluable tool for cell therapy approaches. While differentiation protocols have been developed for cartilage and fibrous connective tissues (e.g., tendon), the signals that regulate the induction and differentiation of human iPSCs toward the AF fate remain unknown.
Methods
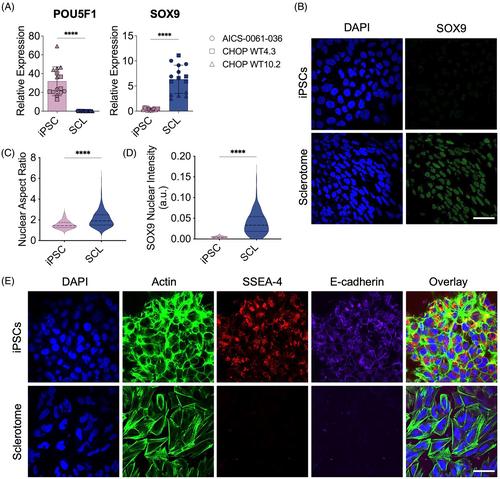
iPSC-derived sclerotome cells were treated with various combinations of developmental signals including transforming growth factor beta 3 (TGF-β3), connective tissue growth factor (CTGF), platelet derived growth factor BB (PDGF-BB), insulin-like growth factor 1 (IGF-1), or the Hedgehog pathway activator, Purmorphamine, and gene expression changes in major AF-associated ECM genes were assessed. The top performing combination treatments were further validated by using three distinct iPSC lines and by assessing the production of upregulated ECM proteins of interest. To conduct a broader analysis of the transcriptomic shifts elicited by each factor combination, and to compare genetic profiles of treated cells to mature human AF cells, a 96.96 Fluidigm gene expression array was applied, and principal component analysis was employed to identify the transcriptional signatures of each cell population and treatment group in comparison to native AF cells.
Results
TGF-β3, in combination with PDGF-BB, CTGF, or IGF-1, induced an upregulation of key AF ECM genes in iPSC-derived sclerotome cells. In particular, treatment with a combination of TGF-β3 with PDGF-BB for 14 days significantly increased gene expression of collagen II and aggrecan and increased protein deposition of collagen I and elastin compared to other treatment groups. Assessment of genes uniquely highly expressed by AF cells or SCL cells, respectively, revealed a shift toward the genetic profile of AF cells with the addition of TGF-β3 and PDGF-BB for 14 days.
Discussion
These findings represent an initial approach to guide human induced pluripotent stem cells toward an AF-like fate for cellular delivery strategies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


