Development of a stable Sf9 insect cell line to produce VSV-G pseudotyped baculoviruses
IF 4.6
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
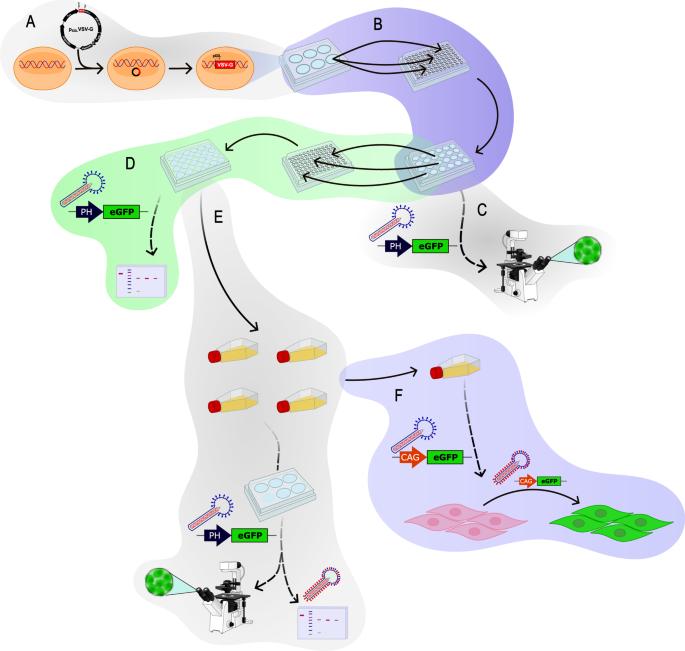
Baculoviruses have shown great potential as gene delivery vectors in mammals, although their effectiveness in transferring genes varies across different cell lines. A widely employed strategy to improve transduction efficiency is the pseudotyping of viral vectors. In this study, we aimed to develop a stable Sf9 insect cell line that inducibly expresses the G-protein of the vesicular stomatitis virus to pseudotype budded baculoviruses. It was obtained by inserting the VSV-G gene under the control of the very strong and infection-inducible pXXL promoter and was subsequently diluted to establish oligoclonal lines, which were selected by the fusogenic properties of VSV-G and its expression levels in infected cells and purified budded virions. Next, to enhance the performance of the cell line, the infection conditions under which functional pseudotyped baculoviruses are obtained were optimized. Finally, different baculoviruses were pseudotyped and the expression of the transgene was quantified in mammalian cells of diverse origins using flow cytometry. The transduction efficiency of pseudotyped baculovirus consistently increased across all tested mammalian cell lines compared with control viruses. These findings demonstrate the feasibility and advantages of improving gene delivery performance without the need to insert the pseudotyping gene into the baculoviral genomes.
开发稳定的 Sf9 昆虫细胞系以生产 VSV-G 假型杆状病毒。
杆状病毒在哺乳动物中作为基因传递载体已显示出巨大的潜力,尽管它们在不同细胞系中传递基因的效果各不相同。提高转导效率的一种广泛采用的策略是对病毒载体进行伪型化。在本研究中,我们旨在开发一种稳定的 Sf9 昆虫细胞系,它能诱导表达水泡性口炎病毒的 G 蛋白,从而伪造出芽杆状病毒。它是通过将 VSV-G 基因插入强感染诱导型 pXXL 启动子控制下获得的,随后稀释建立寡克隆品系,并根据 VSV-G 的致熔特性及其在感染细胞和纯化的芽胞病毒中的表达水平进行筛选。接下来,为了提高细胞系的性能,对获得功能性假型杆状病毒的感染条件进行了优化。最后,对不同的杆状病毒进行了伪型,并利用流式细胞术对不同来源的哺乳动物细胞中转基因的表达进行了量化。与对照病毒相比,伪型杆状病毒在所有测试的哺乳动物细胞系中的转导效率都持续提高。这些发现证明了无需在杆状病毒基因组中插入伪型基因就能提高基因递送性能的可行性和优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


