Total Synthesis of Daphniphyllum Alkaloids: (+)-Daphlongamine E, (+)-Calyciphylline R, and (−)-10-Deoxydaphnipaxianine A
引用次数: 0
Abstract
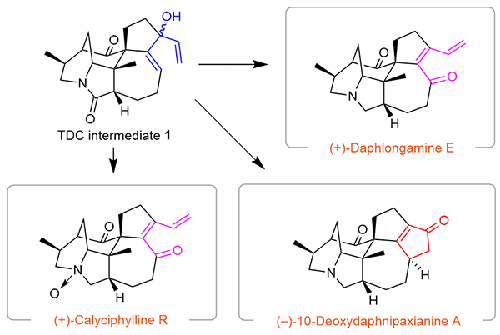
Here, we wish to describe our detailed efforts of the total synthesis of three calyciphylline A-type alkaloids, namely (+)-daphlongamine E, (+)-calyciphylline R, and (−)-10-deoxydaphnipaxianine A. Important steps in our approach include a Pt-catalyzed nitrile hydration, a Babler–Dauben rearrangement, a novel selective amide reduction tactic and, an oxidative Nazarov cyclization via an unfunctionalized tertiary divinyl carbinol (TDC).
Daphniphyllum 生物碱的全合成:(+)-Daphlongamine E、(+)-Calyciphylline R 和 (-)-10-Deoxydaphnipaxianine A
在此,我们希望详细描述一下我们对三种萼片紫碱 A 型生物碱(即 (+)-daphlongamine E、(+)-萼片紫碱 R 和 (-)-10-deoxydaphnipaxianine A)进行全合成的过程。我们研究方法的重要步骤包括铂催化的腈水合反应、巴伯勒-道本重排反应、新型选择性酰胺还原反应以及通过未官能化的叔二乙烯基卡宾醇(TDC)进行氧化纳扎罗夫环化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


