Liver-derived extracellular vesicles improve whole-body glycaemic control via inter-organ communication
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
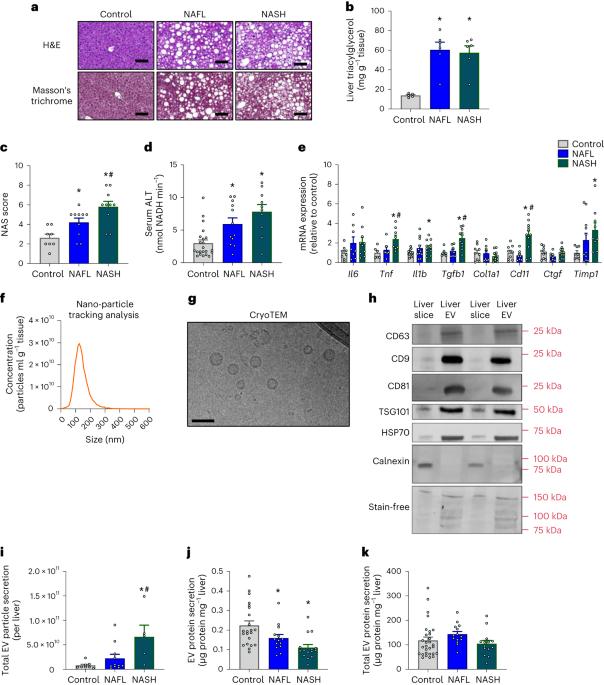
Small extracellular vesicles (EVs) are signalling messengers that regulate inter-tissue communication through delivery of their molecular cargo. Here, we show that liver-derived EVs are acute regulators of whole-body glycaemic control in mice. Liver EV secretion into the circulation is increased in response to hyperglycaemia, resulting in increased glucose effectiveness and insulin secretion through direct inter-organ EV signalling to skeletal muscle and the pancreas, respectively. This acute blood glucose lowering effect occurs in healthy and obese mice with non-alcoholic fatty liver disease, despite marked remodelling of the liver-derived EV proteome in obese mice. The EV-mediated blood glucose lowering effects were recapitulated by administration of liver EVs derived from humans with or without progressive non-alcoholic fatty liver disease, suggesting broad functional conservation of liver EV signalling and potential therapeutic utility. Taken together, this work reveals a mechanism whereby liver EVs act on peripheral tissues via endocrine signalling to restore euglycaemia in the postprandial state. Miotto et al. show that in mice, liver-derived extracellular vesicles act on skeletal muscle and the pancreas and increase glucose effectiveness and insulin secretion, thereby modulating glycaemic control.
肝源性细胞外囊泡通过器官间通信改善全身血糖控制
小型细胞外囊泡(EVs)是一种信号信使,可通过运送其分子货物调节组织间的交流。在这里,我们发现肝源性 EV 是小鼠全身血糖控制的急性调节因子。高血糖时,肝脏分泌到血液循环中的EV会增加,从而通过器官间EV信号直接传递到骨骼肌和胰腺,提高葡萄糖的有效性和胰岛素的分泌。尽管肥胖小鼠的肝源性 EV 蛋白体组发生了明显的重塑,但这种急性血糖降低效应却发生在健康和患有非酒精性脂肪肝的肥胖小鼠身上。给患有或未患有进行性非酒精性脂肪肝的人服用肝脏EV,也能重现EV介导的降血糖效应,这表明肝脏EV信号的功能保持广泛,具有潜在的治疗作用。综上所述,这项研究揭示了肝脏EV通过内分泌信号作用于外周组织以恢复餐后优糖血症的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


