Pharmacogenomic biomarker information on drug labels of the Spanish Agency of Medicines and Sanitary products: evaluation and comparison with other regulatory agencies
IF 2.9
3区 医学
Q2 GENETICS & HEREDITY
引用次数: 0
Abstract
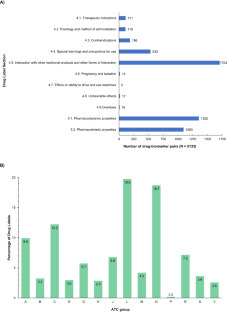
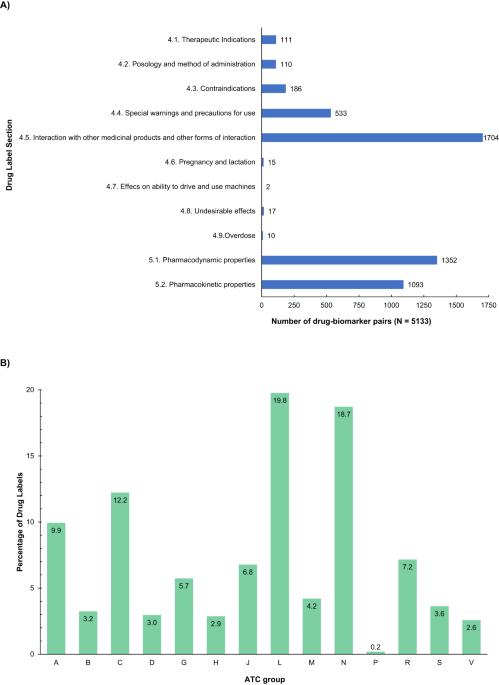
This work aimed to analyse the pharmacogenetic information in the Spanish Drug Regulatory Agency (AEMPS) Summary of Products Characteristics (SmPC), evaluating the presence of pharmacogenetic biomarkers, as well as the associated recommendations. A total of 55.4% of the 1891 drug labels reviewed included information on pharmacogenetic biomarker(s). Pharmacogenomic information appears most frequently in the “antineoplastic and immunomodulating agents”, “nervous system”, and “cardiovascular system” Anatomical Therapeutic Chemical groups. A total of 509 different pharmacogenetic biomarkers were found, of which CYP450 enzymes accounted for almost 34% of the total drug-biomarker associations evaluated. A total of 3679 drug–biomarker pairs were identified, 102 of which were at the 1A level (PharmGKB® classification system), and 33.33% of these drug-pharmacogenetic biomarker pairs were assigned to “actionable PGx”, 12.75% to “informative PGx”, 4.9% to “testing recommended”, and 4.9% to “testing required”. The rate of coincidence in the assigned PGx level of recommendation between the AEMPS and regulatory agencies included in the PharmGKB® Drug Label Annotations database (i.e., the FDA, EMA, SWISS Medic, PMDA, and HCSC) ranged from 45% to 65%, being ‘actionable level’ the most frequent. On the other hand, discrepancies between agencies did not exceed 35%. This study highlights the presence of relevant pharmacogenetic information on Spanish drug labels, which would help avoid interactions, toxicity, or lack of treatment efficacy.
西班牙药品和卫生产品管理局药品标签上的药物基因组生物标记信息:评估以及与其他监管机构的比较
这项研究旨在分析西班牙药品管理局(AEMPS)《产品特征概要》(SmPC)中的药物遗传信息,评估药物遗传生物标志物的存在情况以及相关建议。在接受审查的 1891 个药品标签中,共有 55.4% 包含药物基因生物标记物信息。药物基因组学信息最常出现在 "抗肿瘤和免疫调节剂"、"神经系统 "和 "心血管系统 "解剖治疗化学组中。共发现了 509 种不同的药物基因生物标记物,其中 CYP450 酶占所评估的药物生物标记物关联总数的近 34%。共鉴定出 3679 对药物-生物标记物,其中 102 对属于 1A 级(PharmGKB® 分类系统),这些药物-药物遗传生物标记物中有 33.33% 被归入 "可操作的 PGx",12.75% 被归入 "有参考价值的 PGx",4.9% 被归入 "建议检测",4.9% 被归入 "需要检测"。AEMPS与PharmGKB®药物标签注释数据库中的监管机构(即FDA、EMA、SWISS Medic、PMDA和HCSC)所分配的PGx建议级别的重合率在45%到65%之间,其中 "可操作级别 "最为常见。另一方面,各机构之间的差异不超过 35%。这项研究强调了西班牙药品标签上相关药物遗传学信息的存在,这将有助于避免相互作用、毒性或缺乏疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pharmacogenomics Journal
医学-药学
CiteScore
7.20
自引率
0.00%
发文量
35
审稿时长
6-12 weeks
期刊介绍:
The Pharmacogenomics Journal is a print and electronic journal, which is dedicated to the rapid publication of original research on pharmacogenomics and its clinical applications.
Key areas of coverage include:
Personalized medicine
Effects of genetic variability on drug toxicity and efficacy
Identification and functional characterization of polymorphisms relevant to drug action
Pharmacodynamic and pharmacokinetic variations and drug efficacy
Integration of new developments in the genome project and proteomics into clinical medicine, pharmacology, and therapeutics
Clinical applications of genomic science
Identification of novel genomic targets for drug development
Potential benefits of pharmacogenomics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


