Tissue-resident macrophages exacerbate lung injury after remote sterile damage
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
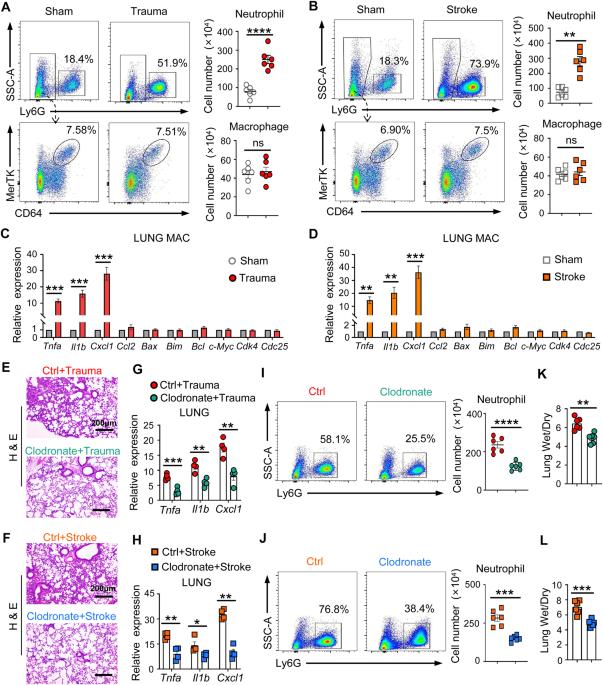
Remote organ injury, which is a common secondary complication of sterile tissue damage, is a major cause of poor prognosis and is difficult to manage. Here, we report the critical role of tissue-resident macrophages in lung injury after trauma or stroke through the inflammatory response. We found that depleting tissue-resident macrophages rather than disrupting the recruitment of monocyte-derived macrophages attenuated lung injury after trauma or stroke. Our findings revealed that the release of circulating alarmins from sites of distant sterile tissue damage triggered an inflammatory response in lung-resident macrophages by binding to receptor for advanced glycation end products (RAGE) on the membrane, which activated epidermal growth factor receptor (EGFR). Mechanistically, ligand-activated RAGE triggered EGFR activation through an interaction, leading to Rab5-mediated RAGE internalization and EGFR phosphorylation, which subsequently recruited and activated P38; this, in turn, promoted RAGE translation and trafficking to the plasma membrane to increase the cellular response to RAGE ligands, consequently exacerbating inflammation. Our study also showed that the loss of RAGE or EGFR expression by adoptive transfer of macrophages, blocking the function of RAGE with a neutralizing antibody, or pharmacological inhibition of EGFR activation in macrophages could protect against trauma- or stroke-induced remote lung injury. Therefore, our study revealed that targeting the RAGE-EGFR signaling pathway in tissue-resident macrophages is a potential therapeutic approach for treating secondary complications of sterile damage.
组织驻留的巨噬细胞会加重远端无菌损伤后的肺损伤。
远端器官损伤是无菌组织损伤的常见继发性并发症,是导致预后不良的主要原因,而且难以控制。在此,我们报告了组织驻留巨噬细胞通过炎症反应在创伤或中风后肺损伤中的关键作用。我们发现,耗竭组织驻留的巨噬细胞而不是破坏单核细胞衍生的巨噬细胞的招募可减轻创伤或中风后的肺损伤。我们的研究结果表明,远处无菌组织损伤部位释放的循环alarmins通过与膜上的高级糖化终产物受体(RAGE)结合,激活表皮生长因子受体(EGFR),从而引发肺驻留巨噬细胞的炎症反应。从机理上讲,配体激活的 RAGE 通过相互作用触发表皮生长因子受体活化,导致 Rab5 介导的 RAGE 内化和表皮生长因子受体磷酸化,进而招募和激活 P38;这反过来又促进了 RAGE 翻译和向质膜的迁移,增加了细胞对 RAGE 配体的反应,从而加剧了炎症。我们的研究还表明,通过收养性转移巨噬细胞、用中和抗体阻断 RAGE 的功能或药物抑制巨噬细胞中表皮生长因子受体的活化来减少 RAGE 或表皮生长因子受体的表达,可以防止创伤或中风引起的远端肺损伤。因此,我们的研究表明,靶向组织驻留巨噬细胞中的RAGE-EGFR信号通路是治疗无菌性损伤继发性并发症的一种潜在治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


