A strategy for producing isotopically labeled peptides with antimicrobial activity or with short in vivo lifetime in Escherichia coli
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Engineered Escherichia coli (E. coli) strains have been widely used to produce isotopically labeled peptides for NMR characterization on their structures and interactions. However, production of antimicrobial peptides (AMPs) by E. coli is still challenging, because AMPs are toxic to E. coli host and would lead to cell death after induction. On the other hand, expression of short peptides in E. coli host often encounter problems of the short in vivo lifetime of the peptides, which were rapidly degraded by endogenous enzymes during expression and purification steps. This report presents a practical method for overcoming these bottlenecks to enable E. coli to express AMPs and peptides that have short in vivo lifetime. This design uses the fusion of thioredoxin tags at both the N- and C-termini of the target peptides. The steric effect of the large soluble tags at both ends of the peptide reduces peptide accessibility, thereby enhancing their in vivo stability and eliminating the toxicity associated with AMPs. The approach was validated using an AMP A3K/L7K-LAH4 (K3K7) and a membrane fusion peptide (FP), which is a segment of the spike protein of SARS-CoV-2 and functions in fusing viral membranes and host cell membranes. Fusion expression of K3K7 with a thioredoxin tag only at the N-terminal resulted in high toxicity to the host cells, leading to impaired cell growth and a failure to obtain expressed fusion protein. In contrast, the fusion proteins from both termini were successfully expressed and purified. In the case of the FP, the fusion of thioredoxin at both termini significantly enhanced its stability, protecting it from enzymatic degradation during expression and purification steps. On the contrary, the FP with thioredoxin fused only at the N-terminal was found to be unstable in E. coli host strains. As stable isotope labeling on peptide is essentially important in NMR-based structure and interaction studies, we also demonstrated that the developed approach enables efficient 15N labeling for NMR studies. This strategy may also be extended to produce other challenging peptides.
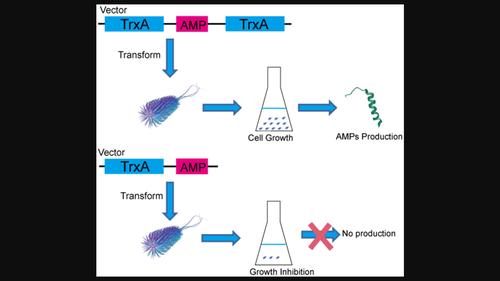
在大肠杆菌中生产具有抗菌活性或体内寿命短的同位素标记肽的策略
工程大肠杆菌(E. coli)菌株已被广泛用于生产同位素标记的肽,以对其结构和相互作用进行核磁共振表征。然而,用大肠杆菌生产抗菌肽(AMPs)仍然具有挑战性,因为 AMPs 对大肠杆菌宿主有毒,诱导后会导致细胞死亡。另一方面,在大肠杆菌宿主中表达短肽往往会遇到短肽在体内寿命短的问题,因为短肽在表达和纯化过程中会被内源酶迅速降解。本报告提出了一种克服这些瓶颈的实用方法,使大肠杆菌能够表达体内寿命短的 AMP 和多肽。这种设计在目标肽的 N 端和 C 端融合了硫代毒素标签。肽两端的大型可溶性标签的立体效应降低了肽的可及性,从而提高了肽在体内的稳定性,并消除了与 AMP 相关的毒性。该方法使用 AMP A3K/L7K-LAH4 (K3K7) 和膜融合肽 (FP) 进行了验证,膜融合肽是 SARS-CoV-2 的尖峰蛋白的一个片段,具有融合病毒膜和宿主细胞膜的功能。K3K7 的融合表达仅在 N 端带有硫代毒素标签,因此对宿主细胞的毒性很高,导致细胞生长受阻,无法获得表达的融合蛋白。相比之下,两个末端的融合蛋白都能成功表达和纯化。就 FP 而言,硫氧还蛋白在两个末端的融合大大提高了其稳定性,使其在表达和纯化步骤中不会被酶降解。相反,只在 N 端融合了硫氧还蛋白的 FP 在大肠杆菌宿主菌株中不稳定。在基于核磁共振的结构和相互作用研究中,肽上稳定的同位素标记非常重要,因此我们也证明了所开发的方法能够为核磁共振研究提供高效的 15N 标记。这种策略也可扩展到其他具有挑战性的多肽的制备中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


