Testosterone nadir and clinical outcomes in patients with advanced prostate cancer: Post hoc analysis of triptorelin pamoate Phase III studies
Abstract
Objective
The objective of the study is to evaluate whether low nadir testosterone during treatment with triptorelin pamoate, a luteinising hormone-releasing hormone (LHRH) agonist, is associated with improved clinical outcomes in patients with advanced prostate cancer using a retrospective analysis of clinical trial data.
Patients and methods
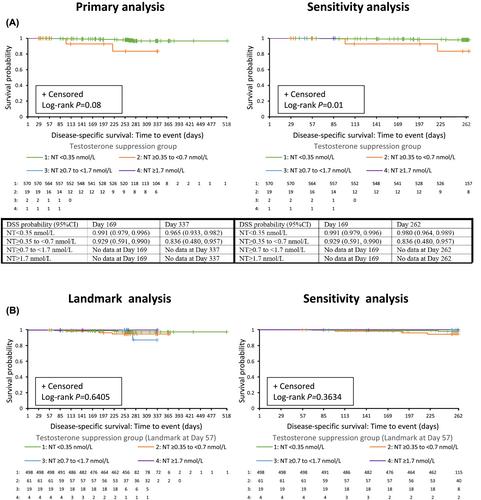
Data were pooled from three prospective, 9–12-month Phase III studies of triptorelin monotherapy in patients with advanced prostate cancer (including NCT00751790). The serum testosterone concentration suppression targets evaluated were <0.35 nmol/L (<10 ng/dl), <0.7 nmol/L (<20 ng/dl), <1.7 nmol/L (<50 ng/dl) and ≥1.7 nmol/L. Overall survival (OS) and disease-specific survival (DSS) by testosterone suppression group were assessed by Kaplan–Meier analysis, with log-rank test. The time frame for the primary analysis was Days 1–518 (median OS follow-up 254 days [range, 29–518 days]) and for the sensitivity analyses was Days 1–262. Supplementary analyses combined the ≥0.7- to <1.7-nmol/L and ≥1.7-nmol/L groups.
Results
The sample size comprised 592 patients (most received triptorelin monotherapy; four reported concomitant androgen receptor-axis–targeted therapy). Nadir testosterones of <0.35, ≥0.35 to <0.7, ≥0.7 to <1.7 and ≥1.7 nmol/L were achieved by 96%, 3.2%, 0.34% and 0.17% of patients, respectively. Better OS with decreasing level of nadir testosterone was observed (p < 0.001) and this persisted after sensitivity/supplemental analyses (all p < 0.001). Differences in DSS with decreasing levels of nadir testosterone were not statistically significant in the primary analysis. Sensitivity/supplemental analysis showed better DSS with decreasing level of nadir testosterone (Days 1–262, p = 0.01; combined groups Days 1–518, p = 0.03; combined groups Days 1–262, p = 0.005).
Conclusion
Low nadir testosterone achieved during treatment with the LHRH agonist triptorelin was associated with improved OS and DSS in patients with advanced prostate cancer.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


