TATB thermal decomposition: Expanding the molecular profile with cryo-focused pyrolysis GC-MS
IF 2
4区 工程技术
Q3 CHEMISTRY, APPLIED
引用次数: 0
Abstract
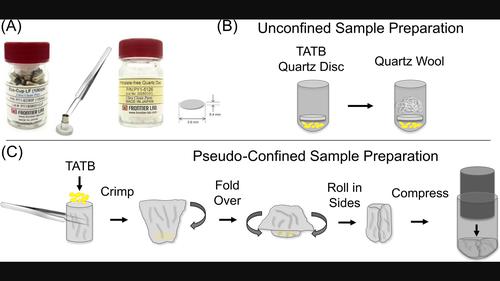
Understanding the molecular composition of high explosives during thermal decomposition is vital for predicting the sensitivity, safety, and performance of explosive materials. The thermal decomposition of 1,3,5-triamino-2,4,6-trinitrobenzene (TATB) has been linked to the formation of furazans through a series of dehydration reactions of the NO2 and NH2 groups on the phenyl ring, along with breakdown into small molecules (≤120 amu). Molecular identification of compounds formed in this transformation of the furazans to light gases has been lacking. To address this, we have applied a pseudo-confined sampling system in a cryo-focused pyrolysis gas chromatography-mass spectrometry (pyGC-MS) system to molecularly identify these intermediates. By design, sublimation of TATB, which has complicated MS analyses of thermal degradation, was significantly reduced and additional compounds were identified with potential structural information. In addition to the known furazan compounds, one of these compounds forms from the loss of oxygen from benzo-trifurazan (F3) and produces an open ring structure that may be the first step in the formation of lower molecular weight furazan breakdown products. The loss of a nitro group from benzo-monofurazan (F1) was also discovered and implicates the formation of oxidizing NO2 gas in the thermal decomposition mechanism. These findings are vital for understanding the proper heat flow from energetic materials on a molecular level, necessary when measuring enthalpy and developing decomposition models based on kinetic parameters.
TATB 热分解:利用低温聚焦热解 GC-MS 扩展分子轮廓
了解热分解过程中烈性炸药的分子组成对于预测爆炸材料的敏感性、安全性和性能至关重要。1,3,5-三氨基-2,4,6-三硝基苯(TATB)的热分解与苯环上的 NO2 和 NH2 基团通过一系列脱水反应形成呋喃有关,同时分解成小分子(≤120 amu)。目前还缺乏对呋喃类化合物向轻质气体转化过程中形成的化合物的分子鉴定。为了解决这个问题,我们在低温聚焦热解气相色谱-质谱(pyGC-MS)系统中应用了一种伪封闭采样系统,对这些中间产物进行分子鉴定。通过设计,使热降解质谱分析复杂化的 TATB 升华现象大大减少,并鉴定出更多具有潜在结构信息的化合物。除了已知的呋喃类化合物外,其中一种化合物是由苯并呋喃三嗪(F3)失去氧而形成的,并产生了一种开放的环状结构,这可能是形成低分子量呋喃分解产物的第一步。此外,还发现苯并单呋咱 (F1) 中的一个硝基脱落,这表明在热分解机制中形成了氧化性二氧化氮气体。这些发现对于了解高能材料在分子水平上的适当热流至关重要,是测量焓值和开发基于动力学参数的分解模型所必需的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Propellants, Explosives, Pyrotechnics
工程技术-工程:化工
CiteScore
4.20
自引率
16.70%
发文量
235
审稿时长
2.7 months
期刊介绍:
Propellants, Explosives, Pyrotechnics (PEP) is an international, peer-reviewed journal containing Full Papers, Short Communications, critical Reviews, as well as details of forthcoming meetings and book reviews concerned with the research, development and production in relation to propellants, explosives, and pyrotechnics for all applications. Being the official journal of the International Pyrotechnics Society, PEP is a vital medium and the state-of-the-art forum for the exchange of science and technology in energetic materials. PEP is published 12 times a year.
PEP is devoted to advancing the science, technology and engineering elements in the storage and manipulation of chemical energy, specifically in propellants, explosives and pyrotechnics. Articles should provide scientific context, articulate impact, and be generally applicable to the energetic materials and wider scientific community. PEP is not a defense journal and does not feature the weaponization of materials and related systems or include information that would aid in the development or utilization of improvised explosive systems, e.g., synthesis routes to terrorist explosives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


