Solvent-Free Synthesis of α-Cyanophosphonates from β-Nitrostyrenes using a Deep Eutectic Solvent
引用次数: 0
Abstract
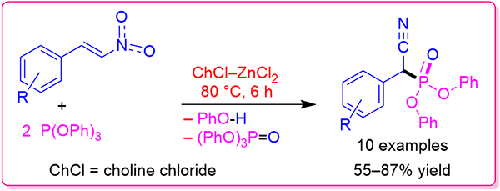
New α-Cyanophosphonates, which are useful reagents for Horner-Wittig reaction, were synthesized in a solvent-free condition, using choline chloride-zinc chloride deep eutectic solvent (DES) as a catalyst. This work is only the second report on the synthesis of these compounds. In the previous report, diethyltrimethylsilylphosphite was used as a reagent and TiCl4 as a catalyst while in this study, both employed reagent and catalyst (triphenyl phosphine and choline chloride-zinc chloride DES) are cheaper, more available and less harmful than the previous work. Moreover, the reaction involves an interesting cascade reaction between β-nitrostyrenes and two equivalents of triphenylphosphite led to the desired product as a new synthetic route. These compounds can be used in the pharmaceutical and agricultural industries, in addition to their synthetic applications in the preparation of α,β-unsaturated nitriles. The reactions were completed using 20 mol% of DES at 80 °C in 6 hours. 10 different β-nitrostyrenes were synthesized in 55-87% yield after purification. β-nitrostyrenes containing electron-donating groups showed higher yields. The reaction was failed when aliphatic and heteroaromatic nitroalkenes and β-nitrostyrenes with electron-withdrawing substituent were employed. Finally, three plausible mechanistic routes were proposed for the reaction, starting from the nucleophilic addition of triphenylphosphite to α-carbon, nitrogen and oxygen atoms.
使用深共晶溶剂从 β-硝基苯炔无溶剂合成 α-氰基膦酸盐
以氯化胆碱-氯化锌深共晶溶剂(DES)为催化剂,在无溶剂条件下合成了可用于霍纳-维蒂希反应的新型α-氰基膦酸盐。这项工作是关于合成这些化合物的第二份报告。在之前的报告中,使用了二乙基三甲基硅基亚磷酸作为试剂和 TiCl4 作为催化剂,而在本研究中,所使用的试剂和催化剂(三苯基磷化氢和氯化胆碱-氯化锌深共晶溶剂 DES)都比之前的工作更便宜、更易获得且危害更小。此外,该反应涉及 β-硝基苯炔与两个当量的亚磷酸三苯酯之间有趣的级联反应,从而获得了所需的产物,这是一条新的合成路线。这些化合物除了可用于制备α,β-不饱和腈类化合物外,还可用于制药和农业。使用 20 mol% 的 DES 在 80 °C、6 小时内完成反应。纯化后合成了 10 种不同的 β-硝基苯炔,收率为 55-87%。含有电子奉献基团的 β-硝基苯炔的产率较高。而当使用脂肪族和杂芳香族硝基烯类以及带有取电子取代基的 β-硝基苯炔时,反应失败。最后,从三苯基亚磷酸与 α 碳原子、氮原子和氧原子的亲核加成开始,为该反应提出了三种可信的机理路线。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


