Experimental and theoretical studies on 1-butyl-3-methyl imidazolium bromine ionic liquids-promoted conversion of aerobic oxidation of cumene
IF 4.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
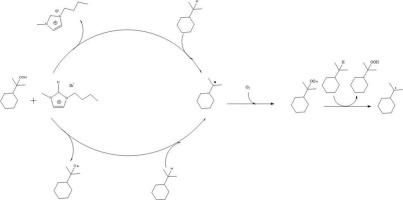
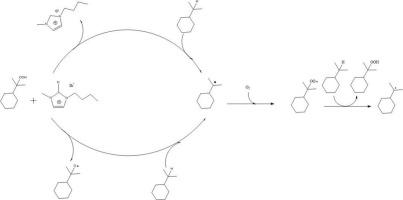
We demonstrated that [Bmim]Br can act as an efficient catalyst exhibiting excellent selectivity in the aerobic oxidation of cumene by decreasing the amount of ILs. The optimal CHP selectivity of 95.4% with 23.6% conversion was obtained from cumene catalyzed by [Bmim]Br at optimal conditions. The kinetic study showed that the reaction follows 1.2-order kinetics. The mechanism study indicated that [Bmim]Br could accelerate the initiation step, transforming the cumene to cumyl radical(R•). The positive effect of hydrogen of imidazolium on the reaction has been observed in the aerobic oxidation of the cumene.
1-丁基-3-甲基咪唑溴离子液体促进积烯有氧氧化转化的实验和理论研究
我们证明,[Bmim]Br 可作为一种高效催化剂,通过减少惰性离子的含量,在积烯的有氧氧化过程中表现出优异的选择性。在最佳条件下,[Bmim]Br 催化积烯获得了 95.4% 的最佳 CHP 选择性和 23.6% 的转化率。动力学研究表明,反应遵循 1.2 阶动力学。机理研究表明,[Bmim]Br 可以加速起始步骤,将积烯转化为积基自由基(R-)。在积烯的有氧氧化过程中,观察到了咪唑鎓的氢对反应的积极影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Communications
化学-物理化学
CiteScore
6.20
自引率
2.70%
发文量
183
审稿时长
46 days
期刊介绍:
Catalysis Communications aims to provide rapid publication of significant, novel, and timely research results homogeneous, heterogeneous, and enzymatic catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


