Immune escape of head and neck cancer mediated by the impaired MHC-I antigen presentation pathway
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Tumor immune evasion is a hallmark of Head and Neck Cancers. The advent of immune checkpoint inhibitors (ICIs) in the first-line setting has transformed the management of these tumors. Unfortunately, the response rate of Head and Neck Squamous Cell Carcinomas (HNSCC) to ICIs is below 15%, regardless of the human papillomavirus (HPV) status, which might be partially related with impaired antigen presentation machinery (APM). Mechanistically, HNSCC cells are usually defective in the expression of MHC-I associated APM, while this transcriptional pathway is critical for the activation of tumor-killing effector T-cells. To specifically illuminate the phenomenon and seek for therapeutic strategies, this review summarizes the most recently identified role of genetic and functional dysregulation of the MHC-I pathway, specifically through changes at the genetic, epigenetic, post-transcriptional, and post-translational levels, which substantially contributes to HNSCC immune escape and ICI resistance. Several treatment modalities can be potentially exploited to restore APM signaling in tumors, which improves anti-tumor immunity through the activation of interferons, vaccines or rimantadine against HPV and the inhibition of EGFR, SHP-2, PI3K and MEK. Additionally, the combinatorial use of radiotherapy or cytotoxic agents with ICIs can synergize to potentiate APM signaling. Future directions would include further dissection of MHC-I related APM signaling in HNSCC and whether reversing this inhibition in combination with ICIs would elicit a more robust immune response leading to improved response rates in HNSCC.
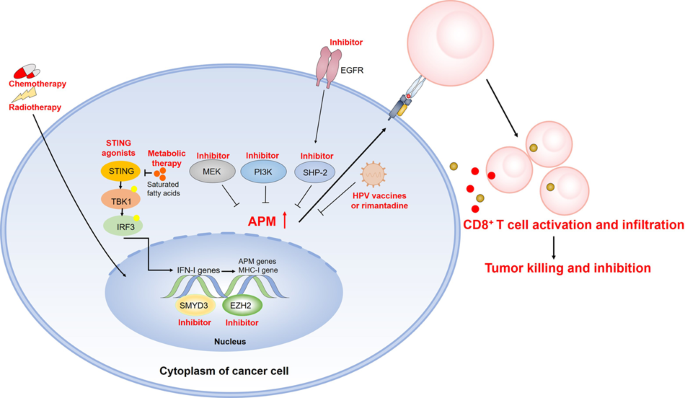
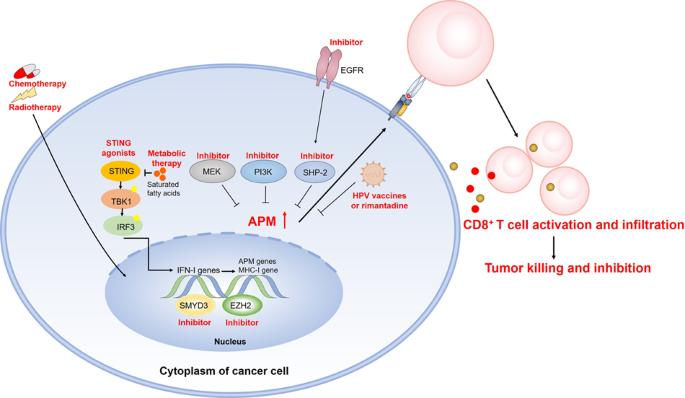
MHC-I抗原递呈途径受损导致的头颈癌免疫逃逸。
肿瘤免疫逃避是头颈癌的一大特征。免疫检查点抑制剂(ICIs)在一线治疗中的出现改变了这些肿瘤的治疗方法。遗憾的是,无论人乳头瘤病毒(HPV)状态如何,头颈部鳞状细胞癌(HNSCC)对 ICIs 的反应率都低于 15%,这可能与抗原递呈机制(APM)受损有部分关系。从机理上讲,HNSCC 细胞通常缺乏与 MHC-I 相关的 APM 表达,而这一转录途径对于激活杀伤肿瘤的效应 T 细胞至关重要。为了具体阐明这一现象并寻求治疗策略,本综述总结了最近发现的 MHC-I 通路遗传和功能失调的作用,特别是通过遗传、表观遗传、转录后和翻译后水平的变化,这在很大程度上导致了 HNSCC 免疫逃逸和 ICI 抗药性。可以利用多种治疗方式来恢复肿瘤中的 APM 信号,通过激活干扰素、疫苗或抗 HPV 的利曼他定以及抑制表皮生长因子受体、SHP-2、PI3K 和 MEK 来提高抗肿瘤免疫力。此外,放疗或细胞毒药物与 ICIs 的联合使用可协同增强 APM 信号传导。未来的研究方向将包括进一步分析 HNSCC 中与 MHC-I 相关的 APM 信号转导,以及结合 ICIs 逆转这种抑制是否会引起更强大的免疫反应,从而提高 HNSCC 的应答率。恢复头颈癌 MHC-I 抗原递呈机制的治疗方法。(红色文字代表相应的策略和结果)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


