Cooperative transcriptional regulation by ATAF1 and HY5 promotes light-induced cotyledon opening in Arabidopsis thaliana
IF 6.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The opening of the embryonic leaves (cotyledons) as seedlings emerge from the dark soil into the light is crucial to ensure the survival of the plant. Seedlings that sprout in the dark elongate rapidly to reach light but keep their cotyledons closed. During de-etiolation, the transition from dark to light growth, elongation slows and the cotyledons open. Here, we report that the transcription factor ACTIVATING FACTOR1 (ATAF1) participates in de-etiolation and facilitates light-induced cotyledon opening. The transition from dark to light rapidly induced ATAF1 expression and ATAF1 accumulation in cotyledons. Seedlings lacking or overexpressing ATAF1 exhibited reduced or enhanced cotyledon opening, respectively, and transcriptomic analysis indicated that ATAF1 repressed the expression of genes associated with growth and cotyledon closure. The activation of the photoreceptor phytochrome A (phyA) by far-red light induced its association with the ATAF1 promoter and stimulation of ATAF1 expression. The transcription factor ELONGATED HYPOCOTYL5 (HY5), which is also activated in response far-red light, cooperated with phyA to induce ATAF1 expression. ATAF1 and HY5 interacted with one another and cooperatively repressed the expression of growth-promoting and cotyledon closure genes. Together, our study reveals a mechanism through which far-red light promotes cotyledon opening.
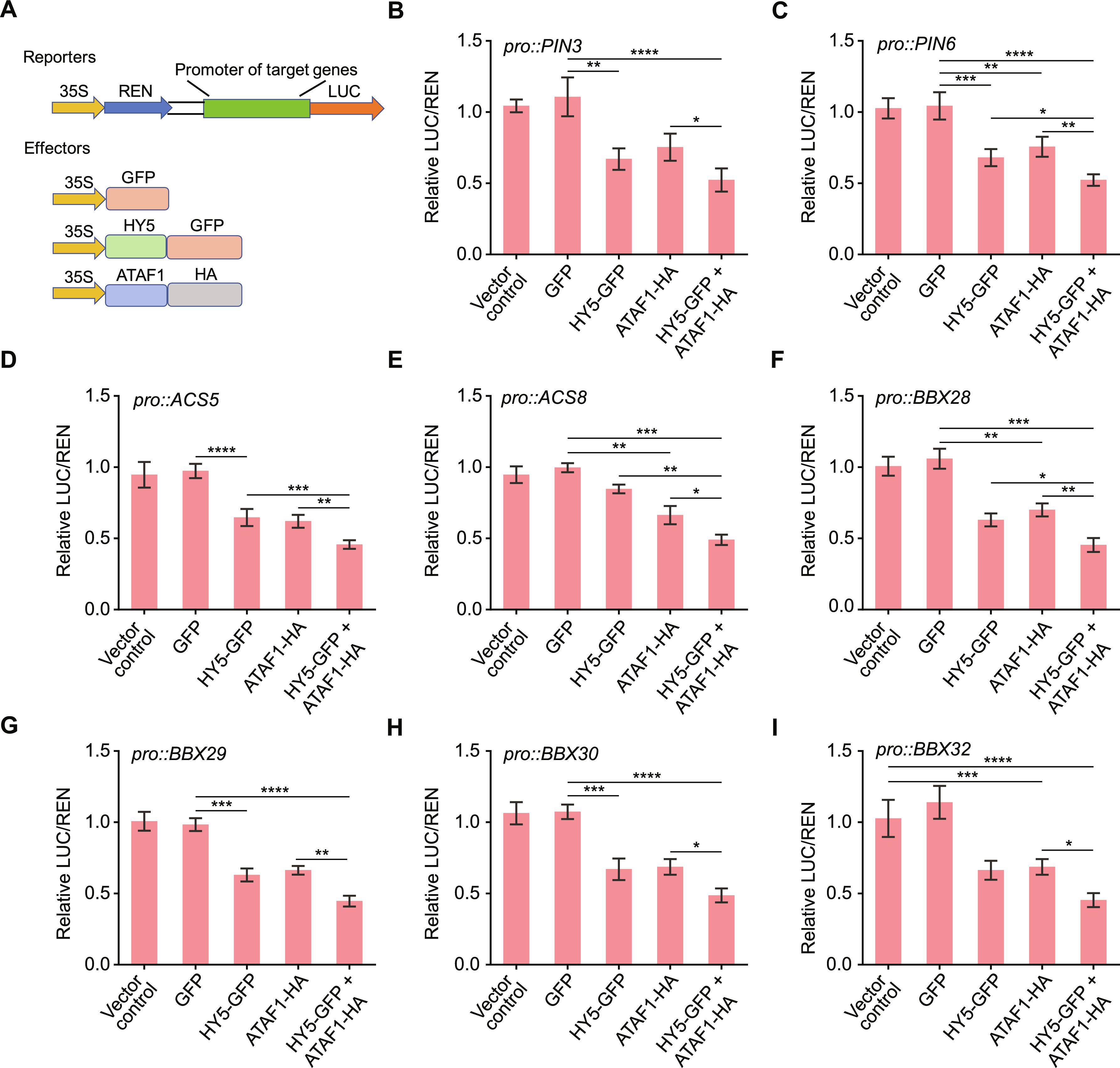
ATAF1 和 HY5 的合作转录调控促进了拟南芥光诱导子叶的开放。
幼苗从黑暗的土壤中萌发到光照下时,胚叶(子叶)的打开对确保植物的存活至关重要。在黑暗中萌发的幼苗会迅速伸长以接触光照,但子叶仍会闭合。在去叶期,即从黑暗生长过渡到光照生长的过程中,伸长速度减慢,子叶张开。在这里,我们报告了转录因子 ACTIVATING FACTOR1(ATAF1)参与去叶期并促进光诱导的子叶打开。从黑暗到光照的转变能迅速诱导 ATAF1 的表达和 ATAF1 在子叶中的积累。转录组分析表明,ATAF1抑制了与生长和子叶闭合相关的基因的表达。远红光激活光感受器植物色素 A(phyA)会诱导其与 ATAF1 启动子结合并刺激 ATAF1 的表达。转录因子 ELONGATED HYPOCOTYL5(HY5)也在远红光下被激活,它与 phyA 合作诱导 ATAF1 的表达。ATAF1 和 HY5 相互作用,共同抑制了促进生长基因和子叶闭合基因的表达。总之,我们的研究揭示了远红光促进子叶开放的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Signaling
BIOCHEMISTRY & MOLECULAR BIOLOGY-CELL BIOLOGY
CiteScore
9.50
自引率
0.00%
发文量
148
审稿时长
3-8 weeks
期刊介绍:
"Science Signaling" is a reputable, peer-reviewed journal dedicated to the exploration of cell communication mechanisms, offering a comprehensive view of the intricate processes that govern cellular regulation. This journal, published weekly online by the American Association for the Advancement of Science (AAAS), is a go-to resource for the latest research in cell signaling and its various facets.
The journal's scope encompasses a broad range of topics, including the study of signaling networks, synthetic biology, systems biology, and the application of these findings in drug discovery. It also delves into the computational and modeling aspects of regulatory pathways, providing insights into how cells communicate and respond to their environment.
In addition to publishing full-length articles that report on groundbreaking research, "Science Signaling" also features reviews that synthesize current knowledge in the field, focus articles that highlight specific areas of interest, and editor-written highlights that draw attention to particularly significant studies. This mix of content ensures that the journal serves as a valuable resource for both researchers and professionals looking to stay abreast of the latest advancements in cell communication science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


