Synthesis of 5,5-Disubstituted N-Methyl-1,3-oxazinanes Containing Monoterpene Fragments
IF 1
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A new method has been developed for the synthesis of 5,5-disubstituted N-methyl-1,3-oxazinanes as a mixture of diastereomers at the 5-position containing monoterpene and branched alkyl substituents in the 5-position. 1,3-Oxazinanes were obtained for the first time by reacting 2,2-substituted 3-aminopropan-1-ols with formaldehyde and sodium borohydride in one stage.
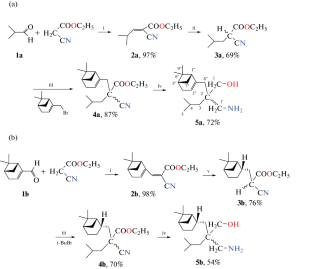

含单萜烯片段的 5,5-二取代 N-甲基-1,3-恶嗪酮的合成
摘要 已开发出一种新方法,用于合成 5,5-二取代的 N-甲基-1,3-恶嗪酮,它是 5 位上含有单萜和支链烷基取代基的非对映异构体混合物。通过将 2,2-取代的 3-氨基丙-1-醇与甲醛和硼氢化钠进行一步反应,首次获得了 1,3-恶嗪酮。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


