Study of the Sorption and Catalytic Properties of Nickel Ferrite with Respect to 2,4-Dinitrophenol
IF 0.6
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
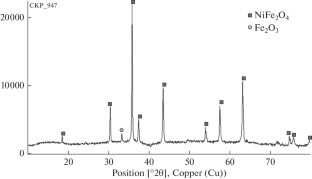
Nanosized NiFe2O4 has been obtained using solution combustion synthesis (SCS). The predominant fraction of the particles has a size of 21–50 nm (TEM). The degree of degradation of 2,4-dinitrophenol in a Fenton-like reaction in the absence of the NiFe2O4 catalyst is 6%, and that in the presence of NiFe2O4 is 53%. Effective oxidative degradation of the pollutant was carried out in a less acidic medium and at a higher initial concentration of dinitrophenol in comparison with the classical Fenton process. The results of the study can be applied in the development of new environmentally friendly water treatment systems at enterprises that use organic dyes in production cycles.
研究铁氧体镍对 2,4-Dinitrophenol 的吸附和催化特性
摘要 利用溶液燃烧合成法(SCS)获得了纳米化的 NiFe2O4。主要颗粒的尺寸为 21-50 纳米(TEM)。在没有 NiFe2O4 催化剂的情况下,2,4-二硝基苯酚在类似芬顿反应中的降解率为 6%,而在有 NiFe2O4 的情况下为 53%。与经典的 Fenton 过程相比,在酸性较低的介质中和较高的二硝基酚初始浓度下,污染物都能得到有效的氧化降解。研究结果可用于在生产循环中使用有机染料的企业开发新的环保型水处理系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

High Energy Chemistry
化学-物理化学
CiteScore
1.50
自引率
28.60%
发文量
62
审稿时长
6-12 weeks
期刊介绍:
High Energy Chemistry publishes original articles, reviews, and short communications on molecular and supramolecular photochemistry, photobiology, radiation chemistry, plasma chemistry, chemistry of nanosized systems, chemistry of new atoms, processes and materials for optical information systems and other areas of high energy chemistry. It publishes theoretical and experimental studies in all areas of high energy chemistry, such as the interaction of high-energy particles with matter, the nature and reactivity of short-lived species induced by the action of particle and electromagnetic radiation or hot atoms on substances in their gaseous and condensed states, and chemical processes initiated in organic and inorganic systems by high-energy radiation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


