Efficacy and toxicity of anlotinib plus camrelizumab versus anlotinib plus S-1 as second-line therapy for advanced esophageal squamous cell carcinoma: A real-world retrospective study
IF 2.8
引用次数: 0
Abstract
Background
No data exist on the efficacy and safety of anlotinib plus camrelizumab doublet as second-line therapy for advanced esophageal squamous cell carcinoma (ESCC). Although anlotinib and the programmed death-1 (PD-1) inhibitor camrelizumab are used as treatments for ESCC, the combined use of anlotinib and camrelizumab as a second-line therapy has not been reported. Therefore, this study explored the efficacy and toxicity of anlotinib plus camrelizumab as second-line therapy for advanced ESCC.
Methods
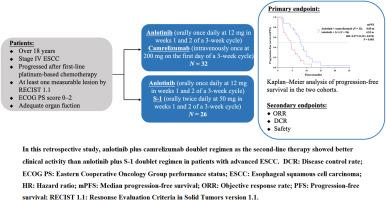
Fifty-eight patients with advanced ESCC undergoing second-line therapy, either with anlotinib plus camrelizumab or anlotinib plus S-1, were enrolled and retrospectively analyzed at Jiangsu Province Hospital of Chinese Medicine from January 2020 to December 2021. The primary endpoint was progression-free survival (PFS), with secondary endpoints including the objective response rate (ORR), disease control rate (DCR), and assessment of toxicity.
Results
In patients with advanced ESCC, the anlotinib plus camrelizumab group (N = 32) exhibited longer PFS (8.00 vs. 4.53 months, P < 0.001), higher ORR (28.1 vs. 19.2%, P = 0.431), and higher DCR (87.5 vs. 65.4%, P = 0.045) than those in the anlotinib plus S-1 group (N = 26). Treatment-related adverse events (TRAEs) were predominantly grade 1/2 in both groups, with a higher incidence of grade 1/2 skin toxicity in patients treated with anlotinib plus camrelizumab (P = 0.033). Two patients (6.3%) developed grade 1/2 immune-related pneumonia. The incidence of grade 3/4 TRAEs did not differ significantly between the two groups. Multivariable Cox regression analysis identified that the drug regimen (P < 0.001), Eastern Cooperative Oncology Group performance status (P = 0.008), and differentiation grade (P = 0.008) were independent prognostic factors for PFS.
Conclusions
Anlotinib plus camrelizumab exhibited promising antitumor efficacy and manageable toxicity when used as a second-line treatment for advanced ESCC.
安罗替尼联合坎瑞珠单抗与安罗替尼联合S-1作为晚期食管鳞状细胞癌二线疗法的疗效和毒性:一项真实世界回顾性研究
背景目前还没有关于安罗替尼加坎瑞珠单抗双药作为晚期食管鳞状细胞癌(ESCC)二线疗法的有效性和安全性的数据。虽然安罗替尼和程序性死亡-1(PD-1)抑制剂坎瑞珠单抗被用作ESCC的治疗方法,但安罗替尼和坎瑞珠单抗联合用作二线疗法的报道还没有。因此,本研究探讨了安罗替尼联合坎瑞珠单抗作为晚期ESCC二线治疗的疗效和毒性。方法对江苏省中医院于2020年1月至2021年12月纳入并回顾性分析的58例接受安罗替尼联合坎瑞珠单抗或安罗替尼联合S-1二线治疗的晚期ESCC患者进行研究。主要终点为无进展生存期(PFS),次要终点包括客观反应率(ORR)、疾病控制率(DCR)和毒性评估。结果在晚期ESCC患者中,安罗替尼加坎瑞珠单抗组(N = 32)比安罗替尼加S-1组(N = 26)表现出更长的PFS(8.00个月对4.53个月,P < 0.001)、更高的ORR(28.1%对19.2%,P = 0.431)和更高的DCR(87.5%对65.4%,P = 0.045)。两组患者的治疗相关不良事件(TRAEs)均以1/2级为主,其中安罗替尼加坎瑞珠单抗组患者的1/2级皮肤毒性发生率更高(P = 0.033)。两名患者(6.3%)出现了1/2级免疫相关肺炎。两组患者的3/4级TRAE发生率无显著差异。多变量Cox回归分析发现,用药方案(P < 0.001)、东部合作肿瘤学组表现状态(P = 0.008)和分化等级(P = 0.008)是PFS的独立预后因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer pathogenesis and therapy
Surgery, Radiology and Imaging, Cancer Research, Oncology
CiteScore
0.80
自引率
0.00%
发文量
0
审稿时长
54 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


