Photocatalyzed C–F Bond Heteroarylation of Trifluoromethylarenes with Heteroarenes: Two Roles of Bu3SnI as Fluoride Ion Scavenger and Activator for Photocatalyst
引用次数: 0
Abstract
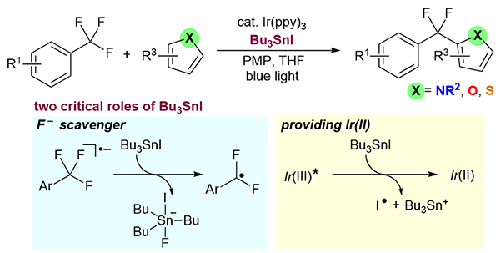
We report the C–F bond heteroarylation of trifluoromethylarenes with heteroarenes by using Ir(ppy)3 catalyst and Bu3SnI under visible-light irradiation. Various heteroarenes such as pyrrole, furan, and thiophene derivatives were applied to the present reaction. The present heteroarylation enables the transformation of various functionalized trifluoromethylarenes because of the mild reaction conditions. Notably, the late-stage transformation of a drug molecule, bicalutamide, was demonstrated. Mechanistic studies including a light on−off interval experiment, Stern–Volmer luminescence-quenching measurements, and DFT calculations clarified two critical roles of Bu3SnI for the successful progress of the heteroarylation. Bu3SnI functions as a fluoride ion scavenger to suppress the undesired C–F bond reformation. Bu3SnI also acts as a single electron source for the reduction of photoexcited Ir(ppy)3* to generate Ir(II) species to effectively reduce ArCF3.
光催化三氟甲基烯与杂环烯的 C-F 键异芳酰化反应:Bu3SnI 作为光催化剂的氟离子清除剂和活化剂的两种作用
我们报告了在可见光辐照下使用 Ir(ppy)3 催化剂和 Bu3SnI 进行三氟甲基烯与杂环烯的 C-F 键杂芳基化反应。吡咯、呋喃和噻吩衍生物等各种杂芳香族化合物都适用于本反应。由于反应条件温和,本发明的杂芳基化反应可实现各种官能化三氟甲基烯的转化。值得注意的是,该反应证明了一种药物分子(比卡鲁胺)的后期转化。包括光开关间隔实验、Stern-Volmer 发光淬灭测量和 DFT 计算在内的机理研究阐明了 Bu3SnI 在杂芳基化成功进行过程中的两个关键作用。Bu3SnI 可充当氟离子清除剂,抑制不希望发生的 C-F 键重构。Bu3SnI 还是光激发 Ir(ppy)3* 生成 Ir(II) 物种以有效还原 ArCF3 的单电子源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


