Distribution and genomic variation of ammonia-oxidizing archaea in abyssal and hadal surface sediments
IF 6.1
Q1 ECOLOGY
引用次数: 0
Abstract
Ammonia-oxidizing archaea of the phylum Thaumarchaeota play a central role in the biogeochemical cycling of nitrogen in benthic sediments, at the interface between pelagic and subsurface ecosystems. However, our understanding of their niche separation and of the processes controlling their population structure in hadal and abyssal surface sediments is still limited. Here, we reconstructed 47 AOA metagenome-assembled genomes (MAGs) from surface sediments of the Atacama and Kermadec trench systems. They formed deep-sea-specific groups within the family Nitrosopumilaceae and were assigned to six amoA gene-based clades. MAGs from different clades had distinct distribution patterns along oxygen-ammonium counter gradients in surface sediments. At the species level, MAGs thus seemed to form different ecotypes and follow deterministic niche-based distributions. In contrast, intraspecific population structure, defined by patterns of Single Nucleotide Variants (SNV), seemed to reflect more complex contributions of both deterministic and stochastic processes. Firstly, the bathymetric range had a strong effect on population structure, with distinct populations in abyssal plains and hadal trenches. Then, hadal populations were clearly separated by trench system, suggesting a strong isolation-by-topography effect, whereas abyssal populations were rather controlled by sediment depth or geographic distances, depending on the clade considered. Interestingly, genetic variability between samples was lowest in sediment layers where the mean MAG coverage was highest, highlighting the importance of selective pressure linked with each AOA clade’s ecological niche. Overall, our results show that deep-sea AOA genome distributions seem to follow both deterministic and stochastic processes, depending on the genomic variability scale considered.
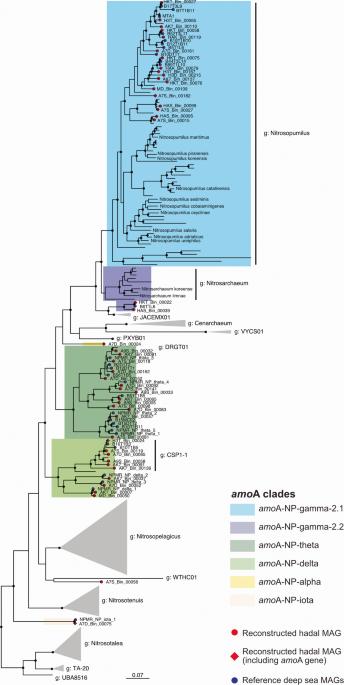
深海和浅海表层沉积物中氨氧化古细菌的分布和基因组变异。
氨氧化古细菌门(Thaumarchaeota)的氨氧化古细菌在底栖沉积物氮的生物地球化学循环中发挥着核心作用,它们处于浮游生态系统和地表下生态系统的交界处。然而,我们对它们在海底和深海表层沉积物中的生态位分离及其种群结构控制过程的了解仍然有限。在这里,我们从阿塔卡马和凯尔马代克海沟系统的表层沉积物中重建了 47 个 AOA 元基因组(MAGs)。它们在亚硝基藻科(Nitrosopumilaceae)中形成了特定的深海群,并被归入六个基于amoA基因的支系。不同支系的 MAGs 在表层沉积物中沿氧-铵反梯度具有不同的分布模式。因此,在物种水平上,MAGs 似乎形成了不同的生态型,并遵循基于生态位的确定性分布。相比之下,由单核苷酸变异(SNV)模式定义的种群内结构似乎反映了更为复杂的决定性和随机过程。首先,水深范围对种群结构有很大影响,在深海平原和海沟有不同的种群。然后,海沟系统明显地将黑线鳕种群分开,这表明地形具有很强的隔离效应,而深海种群则受沉积深度或地理距离的控制,这取决于所考虑的支系。有趣的是,在 MAG 平均覆盖率最高的沉积层中,样本间的遗传变异性最低,这凸显了与每个 AOA 支系生态位相关的选择压力的重要性。总之,我们的研究结果表明,根据所考虑的基因组变异规模,深海 AOA 基因组分布似乎既遵循确定性过程,也遵循随机过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


