The RNA m6A demethylase ALKBH5 drives emergency granulopoiesis and neutrophil mobilization by upregulating G-CSFR expression
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
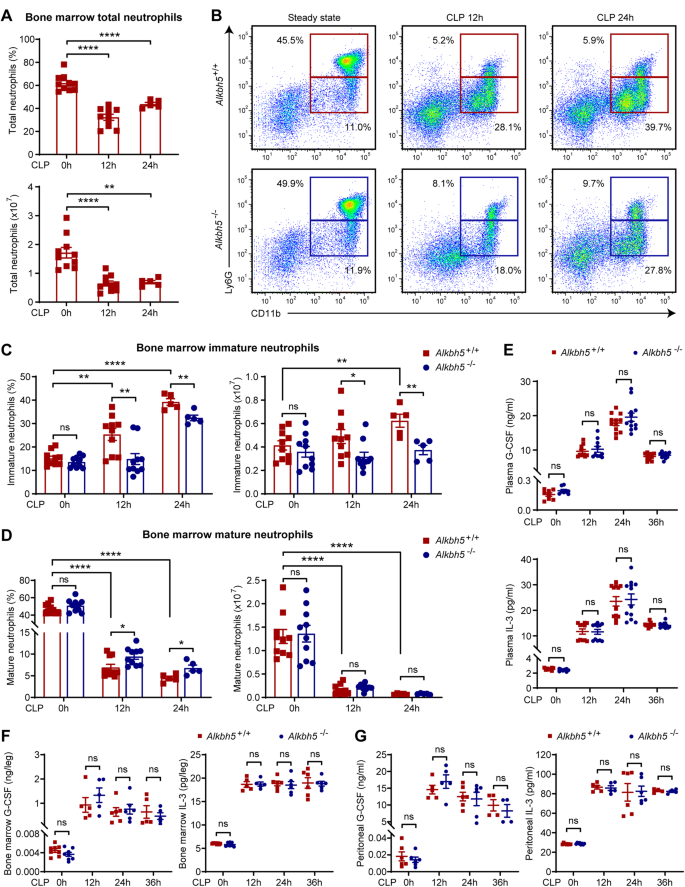
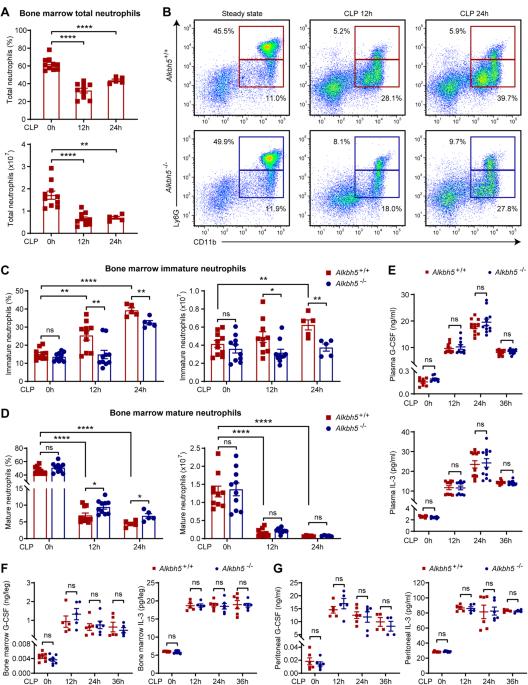
Emergency granulopoiesis and neutrophil mobilization that can be triggered by granulocyte colony-stimulating factor (G-CSF) through its receptor G-CSFR are essential for antibacterial innate defense. However, the epigenetic modifiers crucial for intrinsically regulating G-CSFR expression and the antibacterial response of neutrophils remain largely unclear. N6-methyladenosine (m6A) RNA modification and the related demethylase alkB homolog 5 (ALKBH5) are key epigenetic regulators of immunity and inflammation, but their roles in neutrophil production and mobilization are still unknown. We used cecal ligation and puncture (CLP)-induced polymicrobial sepsis to model systemic bacterial infection, and we report that ALKBH5 is required for emergency granulopoiesis and neutrophil mobilization. ALKBH5 depletion significantly impaired the production of immature neutrophils in the bone marrow of septic mice. In addition, Alkbh5-deficient septic mice exhibited higher retention of mature neutrophils in the bone marrow and defective neutrophil release into the circulation, which led to fewer neutrophils at the infection site than in their wild-type littermates. During bacterial infection, ALKBH5 imprinted production- and mobilization-promoting transcriptome signatures in both mouse and human neutrophils. Mechanistically, ALKBH5 erased m6A methylation on the CSF3R mRNA to increase the mRNA stability and protein expression of G-CSFR, consequently upregulating cell surface G-CSFR expression and downstream STAT3 signaling in neutrophils. The RIP-qPCR results confirmed the direct binding of ALKBH5 to the CSF3R mRNA, and the binding strength declined upon bacterial infection, accounting for the decrease in G-CSFR expression on bacteria-infected neutrophils. Considering these results collectively, we define a new role of ALKBH5 in intrinsically driving neutrophil production and mobilization through m6A demethylation-dependent posttranscriptional regulation, indicating that m6A RNA modification in neutrophils is a potential target for treating bacterial infections and neutropenia.
RNA m6A去甲基化酶ALKBH5通过上调G-CSFR的表达来驱动紧急粒细胞生成和中性粒细胞动员。
粒细胞集落刺激因子(G-CSF)通过其受体 G-CSFR 触发的紧急粒细胞生成和中性粒细胞动员对抗菌先天防御至关重要。然而,对本质上调节 G-CSFR 表达和中性粒细胞抗菌反应至关重要的表观遗传修饰因子在很大程度上仍不清楚。N6-甲基腺苷(m6A)RNA修饰和相关的去甲基化酶alkB同源物5(ALKBH5)是免疫和炎症的关键表观遗传调节因子,但它们在中性粒细胞产生和动员中的作用仍不清楚。我们用盲肠结扎和穿刺(CLP)诱导的多微生物败血症来模拟全身性细菌感染,结果发现ALKBH5是紧急粒细胞生成和中性粒细胞动员所必需的。ALKBH5 的缺失会显著影响败血症小鼠骨髓中未成熟中性粒细胞的生成。此外,与野生型小鼠相比,Alkbh5缺陷败血症小鼠骨髓中成熟中性粒细胞的滞留率更高,而中性粒细胞释放到血液循环中的能力存在缺陷,这导致感染部位的中性粒细胞更少。在细菌感染期间,ALKBH5 在小鼠和人类中性粒细胞中印记了促进生产和动员的转录组特征。从机理上讲,ALKBH5消除了CSF3R mRNA上的m6A甲基化,增加了G-CSFR的mRNA稳定性和蛋白表达,从而上调了细胞表面G-CSFR的表达和中性粒细胞的下游STAT3信号转导。RIP-qPCR 结果证实了 ALKBH5 与 CSF3R mRNA 的直接结合,并且在细菌感染时结合强度下降,这也是细菌感染的中性粒细胞中 G-CSFR 表达下降的原因。综合这些结果,我们确定了 ALKBH5 在通过 m6A 去甲基化依赖性转录后调控内在驱动中性粒细胞生成和动员方面的新作用,表明中性粒细胞中的 m6A RNA 修饰是治疗细菌感染和中性粒细胞减少症的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


