Expectations of duplicate gene retention under the gene duplicability hypothesis
IF 3.4
Q1 Agricultural and Biological Sciences
引用次数: 0
Abstract
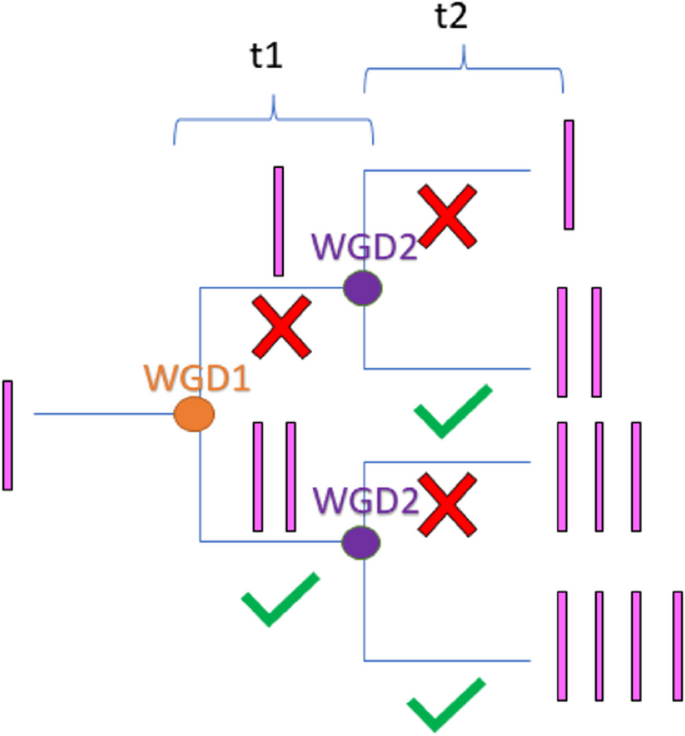
Gene duplication is an important process in evolution. What causes some genes to be retained after duplication and others to be lost is a process not well understood. The most prevalent theory is the gene duplicability hypothesis, that something about the function and number of interacting partners (number of subunits of protein complex, etc.), determines whether copies have more opportunity to be retained for long evolutionary periods. Some genes are also more susceptible to dosage balance effects following WGD events, making them more likely to be retained for longer periods of time. One would expect these processes that affect the retention of duplicate copies to affect the conditional probability ratio after consecutive whole genome duplication events. The probability that a gene will be retained after a second whole genome duplication event (WGD2), given that it was retained after the first whole genome duplication event (WGD1) versus the probability a gene will be retained after WGD2, given it was lost after WGD1 defines the probability ratio that is calculated. Since duplicate gene retention is a time heterogeneous process, the time between the events (t1) and the time since the most recent event (t2) are relevant factors in calculating the expectation for observation in any genome. Here, we use a survival analysis framework to predict the probability ratio for genomes with different values of t1 and t2 under the gene duplicability hypothesis, that some genes are more susceptible to selectable functional shifts, some more susceptible to dosage compensation, and others only drifting. We also predict the probability ratio with different values of t1 and t2 under the mutational opportunity hypothesis, that probability of retention for certain genes changes in subsequent events depending upon how they were previously retained. These models are nested such that the mutational opportunity model encompasses the gene duplicability model with shifting duplicability over time. Here we present a formalization of the gene duplicability and mutational opportunity hypotheses to characterize evolutionary dynamics and explanatory power in a recently developed statistical framework. This work presents expectations of the gene duplicability and mutational opportunity hypotheses over time under different sets of assumptions. This expectation will enable formal testing of processes leading to duplicate gene retention.
基因重复性假说下对重复基因保留的预期
基因复制是进化过程中的一个重要过程。导致一些基因在复制后保留而另一些基因丢失的原因是一个尚不清楚的过程。最流行的理论是基因可复制性假说,即有关相互作用伙伴的功能和数量(蛋白质复合体的亚基数量等)的某些东西,决定了拷贝是否有更多的机会在长进化时期内保留下来。一些基因也更容易受到WGD事件后的剂量平衡效应的影响,使它们更有可能保留更长时间。人们会期望这些影响重复副本保留的过程会影响连续全基因组复制事件后的条件概率比。在第一次全基因组复制事件(WGD1)后保留的基因在第二次全基因组复制事件(WGD2)后保留的概率与在WGD1后丢失的基因在WGD2后保留的概率定义了计算的概率比。由于重复基因保留是一个时间异质性过程,事件之间的时间(t1)和距离最近事件的时间(t2)是计算任何基因组中观察期望的相关因素。在此,我们使用生存分析框架来预测基因可复制假设下不同t1和t2值的基因组的概率比,其中一些基因更容易受到选择性功能转移的影响,一些基因更容易受到剂量补偿的影响,而另一些基因只会漂移。在突变机会假设下,我们还预测了t1和t2不同值的概率比,即某些基因的保留概率在随后的事件中发生变化,这取决于它们先前的保留方式。这些模型是嵌套的,这样突变机会模型就包含了随时间变化的基因可复制性模型。在这里,我们提出了基因可复制性和突变机会假说的形式化,以表征进化动力学和解释力在最近发展的统计框架。这项工作提出了期望的基因可复制性和突变机会假设随时间在不同的假设集。这一期望将使导致重复基因保留过程的正式测试成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

BMC Evolutionary Biology
生物-进化生物学
CiteScore
5.80
自引率
0.00%
发文量
0
审稿时长
6 months
期刊介绍:
BMC Evolutionary Biology is an open access, peer-reviewed journal that considers articles on all aspects of molecular and non-molecular evolution of all organisms, as well as phylogenetics and palaeontology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


