Effectiveness of the T-Control catheter: A study protocol
Abstract
Background
Foley catheters have been subject to limited development in the last few decades. They fulfil their basic function of draining urine from the bladder but cause other associated problems. T-Control is a new silicone Foley catheter with an integrated fluid control valve whose design aims to reduce the risks associated with bladder catheterisation by a multifactorial approach. The general purpose of this study is to evaluate the effectiveness and cost-effectiveness of the T-Control catheter versus the Foley-type catheter in patients with Acute Urine Retention (AUR).
Study design
This is a pragmatic, open, multicentre, controlled clinical trial with random allocation to the T-Control catheter or a conventional Foley-type catheter in patients with AUR.
Endpoints
The magnitude of infections will be analysed as a primary endpoint. While as secondary endpoint, the following will be analysed: rate of symptomatic and asymptomatic infections; days free of infection; quality of life-related to self-perceived health; indication of associated antibiotic treatments; determination of biofilm; number of catheter-related adverse events; use of each type of catheterisation's healthcare resources; level of satisfaction and workload of health professionals and acceptability of the T-Control device as well as the patient experience.
Patients and methods
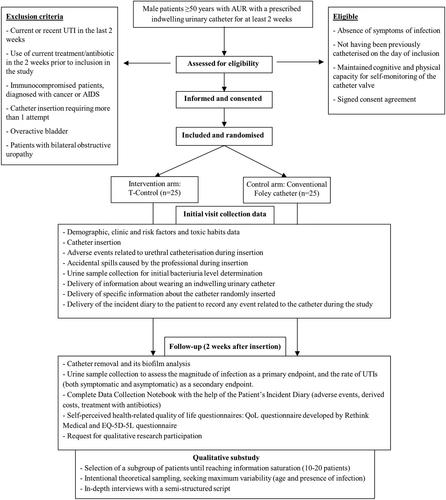
Eligible patients are male adults aged ≥50 years, with AUR and with an indication of bladder catheterisation for at least 2 weeks. The estimated sample size is 50 patients. Patient follow-up includes both the time of catheter insertion and its removal or change 2 weeks later, plus 2 weeks after this time when the patient will be called for an in-depth interview.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


