HGF facilitates methylation of MEG3, potentially implicated in vemurafenib resistance in melanoma
Abstract
Background
Melanoma, a frequently encountered cutaneous malignancy characterized by a poor prognosis, persists in presenting formidable challenges despite the advancement in molecularly targeted drugs designed to improve survival rates significantly. Unfortunately, as more therapeutic choices have developed over time, the gradual emergence of drug resistance has become a notable impediment to the effectiveness of these therapeutic interventions. The hepatocyte growth factor (HGF)/c-met signaling pathway has attracted considerable attention, associated with drug resistance stemming from multiple potential mutations within the c-met gene. The activation of the HGF/c-met pathway operates in an autocrine manner in melanoma. Notably, a key player in the regulatory orchestration of HGF/c-met activation is the long non-coding RNA MEG3.
Methods
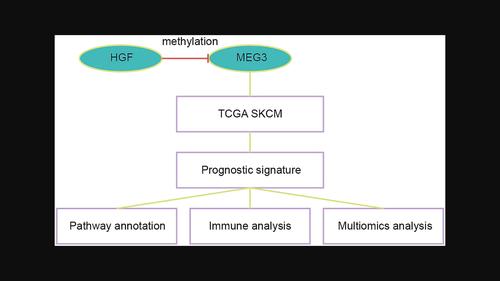
Melanoma tissues were collected to measure MEG3 expression. In vitro validation was performed on MEG3 to prove its oncogenic roles. Bioinformatic analyses were conducted on the TCGA database to build the MEG3-related score. The immune characteristics and mutation features of the MEG3-related score were explored.
Results
We revealed a negative correlation between HGF and MEG3. In melanoma cells, HGF inhibited MEG3 expression by augmenting the methylation of the MEG3 promoter. Significantly, MEG3 exhibits a suppressive impact on the proliferation and migration of melanoma cells, concurrently inhibiting c-met expression. Moreover, a predictive model centered around MEG3 demonstrates notable efficacy in forecasting critical prognostic indicators, immunological profiles, and mutation statuses among melanoma patients.
Conclusions
The present study highlights the potential of MEG3 as a pivotal regulator of c-met, establishing it as a promising candidate for targeted drug development in the ongoing pursuit of effective therapeutic interventions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


