Physiologically based pharmacokinetic (PBPK) modeling to predict the pharmacokinetics of irbesartan in different CYP2C9 genotypes
Abstract
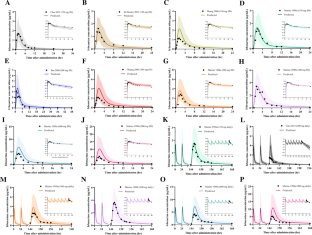
Irbesartan, a potent and selective angiotensin II type-1 (AT1) receptor blocker (ARB), is one of the representative medications for the treatment of hypertension. Cytochrome P450 (CYP) 2C9 is primarily involved in the oxidation of irbesartan. CYP2C9 is highly polymorphic, and genetic polymorphism of this enzyme is the leading cause of significant alterations in the pharmacokinetics of irbesartan. This study aimed to establish the physiologically based pharmacokinetic (PBPK) model to predict the pharmacokinetics of irbesartan in different CYP2C9 genotypes. The irbesartan PBPK model was established using the PK-Sim® software. Our previously reported pharmacogenomic data for irbesartan was leveraged in the development of the PBPK model and collected clinical pharmacokinetic data for irbesartan was used for the validation of the model. Physicochemical and ADME properties of irbesartan were obtained from previously reported data, predicted by the modeling software, or optimized to fit the observed plasma concentration–time profiles. Model evaluation was performed by comparing the predicted plasma concentration–time profiles and pharmacokinetic parameters to the observed results. Predicted plasma concentration–time profiles were visually similar to observed profiles. Predicted AUCinf in CYP2C9*1/*3 and CYP2C9*1/*13 genotypes were increased by 1.54- and 1.62-fold compared to CYP2C9*1/*1 genotype, respectively. All fold error values for AUC and Cmax in non-genotyped and CYP2C9 genotyped models were within the two-fold error criterion. We properly established the PBPK model of irbesartan in different CYP2C9 genotypes. It can be used to predict the pharmacokinetics of irbesartan for personalized pharmacotherapy in individuals of various races, ages, and CYP2C9 genotypes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


