Encapsulation of Cofacial Diarylacetylene Dimers Using [
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
[c2]Daisy chain rotaxanes are mechanically interlocked molecules that can cofacially assemble two π-conjugated molecules. In this study, we employed a rotaxane architecture to encapsulate diarylacetylene dimers modified with an electron-withdrawing or electron-donating substituent in two permethylated α-cyclodextrins. Solvent- and concentration-dependent 1H nuclear magnetic resonance measurements indicated the selective formation of the cofacial dimers. These dimers were mechanically interlocked by condensation with 4-{2-[2-(2-hydroxyethoxy)ethoxy]ethoxy}-3,5-dimethylaniline to obtain the corresponding [c2]daisy chain rotaxanes (up to 68% yield). UV-visible absorption and fluorescence spectra revealed that the diarylacetylene substituents modulated the optical properties of the rotaxanes.
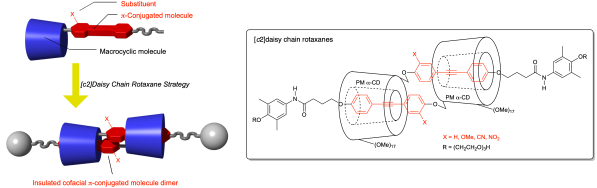
用[]包封共面二芳基乙炔二聚体
[c2]菊花链轮烷是一种机械互锁的分子,可以将两个π共轭分子共面组装。在这项研究中,我们采用轮烷结构封装了两个过甲基化α-环糊精中由吸电子或供电子取代基修饰的二芳基乙炔二聚体。溶剂和浓度相关的1H核磁共振测量表明共面二聚体的选择性形成。这些二聚体通过与4-{2-[2-(2-羟基乙氧基)乙氧基]乙氧基}-3,5-二甲基苯胺缩合得到相应的[c2]菊花链轮烷(产率高达68%)。紫外-可见吸收光谱和荧光光谱显示,二芳基乙炔取代基调节了轮烷的光学性质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heterocycles
化学-有机化学
CiteScore
1.50
自引率
0.00%
发文量
108
审稿时长
1 months
期刊介绍:
Since its inception in 1973 HETEROCYCLES has provided a platform for the rapid exchange of research in the areas of organic, pharmaceutical, analytical, and medicinal chemistry of heterocyclic compounds in addition to communications, papers, reviews, a special section of the journal presents newly-discovered natural products whose structure has recently been established.
Another section is devoted to the total synthesis of previously documented natural products with heterocyclic ring systems.
Due to the fact that the journal is able to publish articles within two months of receipt of the manuscripts, researchers in this field can obtain up-to-date information on heterocyclic research by reading Heterocycles regularly.
Audience: Organic and Physical Organic Chemists, Biochemists, Pharmacologists and Scientists studying heterocyclic compounds
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


