Synthesis of Protected L,L-Cyclotryptophyltyrosine, a Key Unit of Tryptorubin A
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A protected form of L,L-cyclotryptophyltyrosine, a key structure of the bicyclic hexapeptide tryptorubin A isolated from Streptomyces sp. CLI2509, was synthesized. Treatment of Boc-L-Trp-3-borono-L-Tyr(Me)-OMe, obtained from the coupling reaction of Boc-L-Trp-OH and H2N-3-iodo-L-Tyr(Me)-OMe and the subsequent borylation of iodide, with Cu(OAc)2, pyridine, and 4 Å molecular sieves in dichloromethane at 30 °C for 48 h at a concentration of 0.001 M gave protected L,L-cyclotryptophyltyrosine in 58% yield. The structure of obtained protected L,L-cyclotryptophyltyrosine was confirmed by X-ray crystallography, revealing the presence of a pyramidal indole nitrogen atom.
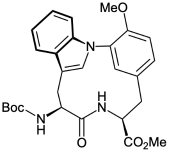
色氨酸a关键单元保护性L-环色氨酸的合成
合成了链霉菌(Streptomyces sp. CLI2509)双环六肽色氨酸A的关键结构L-环色氨酸的保护形式。Boc-L-Trp-3-borono-L-Tyr(Me)-OMe是由Boc-L-Trp-OH和h2n -3-碘-L- tyr (Me)-OMe偶联反应得到的,随后碘化物与Cu(OAc)2、吡啶和4个Å分子筛在30°C的二氯甲烷中处理48 h,浓度为0.001 M,得到保护的L,L-环色氨酸,产率为58%。用x射线晶体学证实了得到的受保护L,L-环色氨酸的结构,发现存在一个锥体吲哚氮原子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heterocycles
化学-有机化学
CiteScore
1.50
自引率
0.00%
发文量
108
审稿时长
1 months
期刊介绍:
Since its inception in 1973 HETEROCYCLES has provided a platform for the rapid exchange of research in the areas of organic, pharmaceutical, analytical, and medicinal chemistry of heterocyclic compounds in addition to communications, papers, reviews, a special section of the journal presents newly-discovered natural products whose structure has recently been established.
Another section is devoted to the total synthesis of previously documented natural products with heterocyclic ring systems.
Due to the fact that the journal is able to publish articles within two months of receipt of the manuscripts, researchers in this field can obtain up-to-date information on heterocyclic research by reading Heterocycles regularly.
Audience: Organic and Physical Organic Chemists, Biochemists, Pharmacologists and Scientists studying heterocyclic compounds
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


