Synthesis and Properties of Novel Thiophene Polycyclic Aromatic Hydrocarbons Based on Pyrene and Their Radical Cations
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
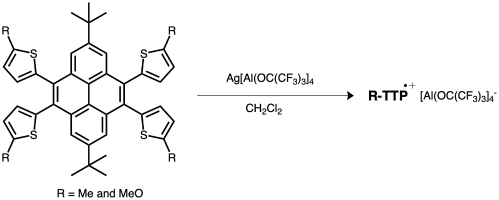
Two new thiophene-modified pyrene derivatives, 4,5,9,10-tetra- (2-(5-methyl)thienyl)-2,7-di-tert-butylpyrene (1) and 4,5,9,10-tetra- (2-(5-methoxy)thienyl)-2,7-di-tert-butylpyrene (2), were designed and synthesized via Friedel–Crafts alkylation and Stille coupling reactions of pyrene. The stable monoradical cations 1•+ and 2•+ based on the thiophene-modified pyrene derivatives were generated by the one-electron oxidation of the pyrene-based thiophene polycyclic aromatic groups with Ag[Al(ORF)4] (ORF = OC(CF3)3). Their structures and properties were investigated using 1H nuclear magnetic resonance spectroscopy, ultraviolet–visible spectrophotometry, X-ray diffraction, density functional theory calculations, and electron paramagnetic resonance spectroscopy. The electron spin density was mainly concentrated on the pyrene ring, with a minor spillover to the peripheral thienyl moieties. Compound 1•+, which is the first reported monoradical cation of thiophene-modified pyrene, is expected to have broad application prospects in the fields of semiconductors and optoelectronic materials.
基于芘及其自由基阳离子的新型噻吩多环芳烃的合成与性质
通过对芘的Friedel-Crafts烷基化和Stille偶联反应,设计并合成了两个新的噻吩修饰的芘衍生物4,5,9,10-四-(2-(5-甲基)噻基)-2,7-二叔丁基芘(1)和4,5,9,10-四-(2-(5-甲氧基)噻基)-2,7-二叔丁基芘(2)。通过与Ag[Al(ORF)4] (ORF = OC(CF3)3)的单电子氧化反应,得到了噻吩修饰芘衍生物上稳定的单自由基1•+和2•+。采用1H核磁共振波谱、紫外可见分光光度计、x射线衍射、密度泛函理论计算和电子顺磁共振波谱对其结构和性质进行了研究。电子自旋密度主要集中在芘环上,对周围的噻基部分有少量溢出。化合物1•+是首次报道的噻吩修饰芘的单自由基阳离子,有望在半导体、光电材料等领域具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Heterocycles
化学-有机化学
CiteScore
1.50
自引率
0.00%
发文量
108
审稿时长
1 months
期刊介绍:
Since its inception in 1973 HETEROCYCLES has provided a platform for the rapid exchange of research in the areas of organic, pharmaceutical, analytical, and medicinal chemistry of heterocyclic compounds in addition to communications, papers, reviews, a special section of the journal presents newly-discovered natural products whose structure has recently been established.
Another section is devoted to the total synthesis of previously documented natural products with heterocyclic ring systems.
Due to the fact that the journal is able to publish articles within two months of receipt of the manuscripts, researchers in this field can obtain up-to-date information on heterocyclic research by reading Heterocycles regularly.
Audience: Organic and Physical Organic Chemists, Biochemists, Pharmacologists and Scientists studying heterocyclic compounds
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


