Circulating extracellular choline acetyltransferase regulates inflammation
Abstract
Background
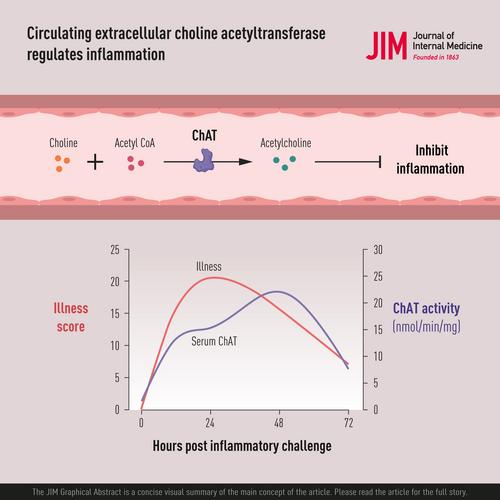
Choline acetyltransferase (ChAT) is required for the biosynthesis of acetylcholine, the molecular mediator that inhibits cytokine production in the cholinergic anti-inflammatory pathway of the vagus nerve inflammatory reflex. Abundant work has established the biology of cytoplasmic ChAT in neurons, but much less is known about the potential presence and function of ChAT in the extracellular milieu.
Objectives
We evaluated the hypothesis that extracellular ChAT activity responds to inflammation and serves to inhibit cytokine release and attenuate inflammation.
Methods
After developing novel methods for quantification of ChAT activity in plasma, we determined whether ChAT activity changes in response to inflammatory challenges.
Results
Active ChAT circulates within the plasma compartment of mice and responds to immunological perturbations. Following the administration of bacterial endotoxin, plasma ChAT activity increases for 12–48 h, a time period that coincides with declining tumor necrosis factor (TNF) levels. Further, a direct activation of the cholinergic anti-inflammatory pathway by vagus nerve stimulation significantly increases plasma ChAT activity, whereas the administration of bioactive recombinant ChAT (r-ChAT) inhibits endotoxin-stimulated TNF production and anti-ChAT antibodies exacerbate endotoxin-induced TNF levels, results of which suggest that ChAT activity regulates endogenous TNF production. Administration of r-ChAT significantly attenuates pro-inflammatory cytokine production and disease activity in the dextran sodium sulfate preclinical model of inflammatory bowel disease. Finally, plasma ChAT levels are also elevated in humans with sepsis, with the highest levels observed in a patient who succumbed to infection.
Conclusion
As a group, these results support further investigation of ChAT as a counter-regulator of inflammation and potential therapeutic agent.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


