Effectiveness of SARS-CoV-2 testing strategies: A scoping review
Abstract
Introduction
Rapid identification of severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infections by testing potentially reduced coronavirus disease-19 (COVID-19) cases. Testing strategies varied across countries and during different stages of the pandemic. This scoping review aims to map the available evidence on the effectiveness of SARS-CoV-2 testing strategies for suspected cases and asymptomatic populations to inform the development of World Health Organization recommendations for SARS-CoV-2 testing strategies.
Methods
We followed the standard methods for scoping reviews. We searched Medline (OVID), EMBASE (Elsevier), and Europe PMC using a comprehensive search strategy. The search was conducted in January 2023 and covered the period from January 2020 to January 2023. Two review authors independently screened the titles and abstracts, and full texts. Data were extracted onto a pilot-tested form by a review author and cross-checked by another review author. We provided a descriptive report summarizing the extracted data around the outcomes and created an interactive map of the available evidence using the evidence for policy and practice mapper.
Results
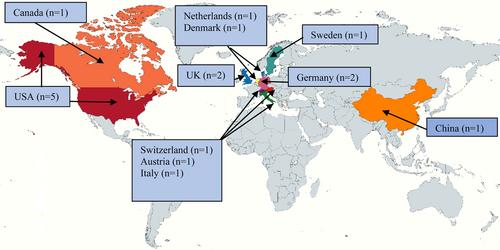
We identified 34,550 citations from the databases. After the screening, we included 17 studies from 11 countries for data extraction. The study designs were randomized controlled trials (n = 3), nonrandomized experimental studies (n = 3), cohort studies (n = 3), cross-sectional studies (n = 4), self-controlled case series (n = 1), and economic evaluations (n = 3). Among the included studies, 14 used reverse transcription-polymerase chain reaction and 10 studies used antigen-detecting rapid diagnostic test. The settings of the studies were healthcare facilities (n = 8), communities (n = 4), schools, and workplaces (n = 3). Included studies considered symptomatic and asymptomatic individuals, or both, or asymptomatic contacts. Most of the studies (n = 14) reported the COVID-19 positivity rate as the primary outcome. Other reported outcomes are the number of COVID-19 cases (n = 11), number of hospitalizations and deaths (n = 3), and cost (n = 3).
Conclusion
We identified evidence gaps in the effectiveness of SARS-CoV-2 testing strategies, particularly in specific settings such as schools and long-term care facilities. This scoping review provides a foundation for further research, allowing researchers and stakeholders to focus on addressing the identified gaps.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


