Improving thymus implantation for congenital athymia with interleukin-7
Abstract
Objectives
Thymus implantation is a recently FDA-approved therapy for congenital athymia. Patients receiving thymus implantation develop a functional but incomplete T cell compartment. Our objective was to develop a mouse model to study clinical thymus implantation in congenital athymia and to optimise implantation procedures to maximise T cell education and expansion of naïve T cells.
Methods
Using Foxn1nu athymic mice as recipients, we tested MHC-matched and -mismatched donor thymi that were implanted as fresh tissue or cultured to remove donor T cells. We first implanted thymus under the kidney capsule and then optimised intramuscular implantation. Using competitive adoptive transfer assays, we investigated whether the failure of newly developed T cells to expand into a complete T cell compartment was because of intrinsic deficits or whether there were deficits in engaging MHC molecules in the periphery. Finally, we tested whether recombinant IL-7 would promote the expansion of host naïve T cells educated by the implanted thymus.
Results
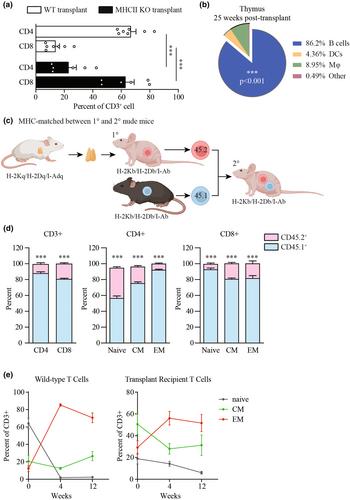
We determined that thymus implants in Foxn1nu athymic mice mimic many aspects of clinical thymus implants in patients with congenital athymia. When we implanted cultured, MHC-mismatched donor thymus into Foxn1nu athymic mice, mice developed a limited T cell compartment with notably underdeveloped naïve populations and overrepresented memory-like T cells. Newly generated T cells were predominantly educated by MHC molecules expressed by the donor thymus, thus potentially undergoing another round of selection once in the peripheral circulation. Using competitive adoptive transfer assays, we compared expansion rates of T cells educated on donor thymus versus T cells educated during typical thymopoiesis in MHC-matched and -mismatched environments. Once in the circulation, regardless of the MHC haplotypes, T cells educated on a donor thymus underwent abnormal expansion with initially more robust proliferation coupled with greater cell death, resembling IL-7 independent spontaneous expansion. Treating implanted mice with recombinant interleukin (IL-7) promoted homeostatic expansion that improved T cell development, expanded the T cell receptor repertoire, and normalised the naïve T cell compartment.
Conclusion
We conclude that implanting cultured thymus into the muscle of Foxn1nu athymic mice is an appropriate system to study thymus implantation for congenital athymia and immunodeficiencies. T cells are educated by the donor thymus, yet naïve T cells have deficits in expansion. IL-7 greatly improves T cell development after thymus implantation and may offer a novel strategy to improve outcomes of clinical thymus implantation.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


