Synthesis and Sorption Properties of Lithium Aluminosilicate
IF 0.8
4区 材料科学
Q3 METALLURGY & METALLURGICAL ENGINEERING
Protection of Metals and Physical Chemistry of Surfaces
Pub Date : 2023-11-24
DOI:10.1134/S2070205123701046
引用次数: 0
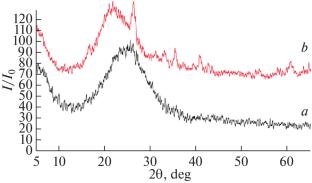
Abstract—The article presents data on the synthesis of nanostructured, X-ray amorphous lithium aluminosilicate, with a Si : Al ratio of 3 : 1. The composition, morphology, and thermal behavior were studied. The sorption isotherm of Cs+ ions was obtained under static conditions with a ratio of T : L = 1 : 400. The maximum sorption capacity, degree of extraction, and distribution coefficients of cesium were determined. Data on the sorption kinetics of Cs+ ions were obtained at temperatures 30 and 60°C, and the activation energy of the sorption process and diffusion coefficients were calculated.
硅酸铝锂的合成及吸附性能研究
摘要:本文介绍了Si: Al比为3:1的纳米结构、x射线无定形硅酸铝锂的合成。对其组成、形貌和热行为进行了研究。在T: L = 1: 400的静态条件下,得到了Cs+离子的吸附等温线。测定了铯的最大吸附量、萃取度和分配系数。在温度为30℃和60℃时获得了Cs+离子的吸附动力学数据,并计算了吸附过程的活化能和扩散系数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.90
自引率
18.20%
发文量
90
审稿时长
4-8 weeks
期刊介绍:
Protection of Metals and Physical Chemistry of Surfaces is an international peer reviewed journal that publishes articles covering all aspects of the physical chemistry of materials and interfaces in various environments. The journal covers all related problems of modern physical chemistry and materials science, including: physicochemical processes at interfaces; adsorption phenomena; complexing from molecular and supramolecular structures at the interfaces to new substances, materials and coatings; nanoscale and nanostructured materials and coatings, composed and dispersed materials; physicochemical problems of corrosion, degradation and protection; investigation methods for surface and interface systems, processes, structures, materials and coatings. No principe restrictions exist related systems, types of processes, methods of control and study. The journal welcomes conceptual, theoretical, experimental, methodological, instrumental, environmental, and all other possible studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


