TRIM3 facilitates ferroptosis in non-small cell lung cancer through promoting SLC7A11/xCT K11-linked ubiquitination and degradation
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
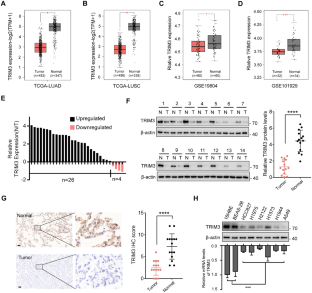
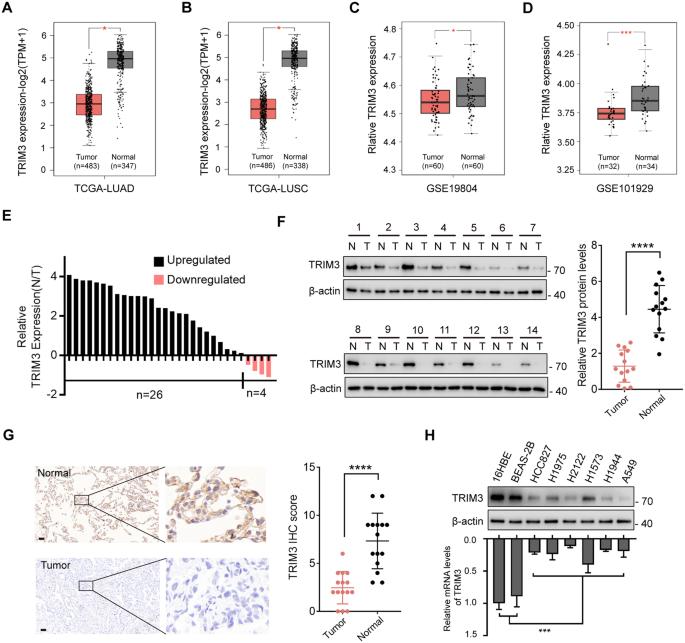
Ferroptosis, a unique form of regulated necrotic cell death, is caused by excessive iron-dependent lipid peroxidation. However, the underlying mechanisms driving ferroptosis in human cancers remain elusive. In this study, we identified TRIM3, an E3 ubiquitin-protein ligase, as a key regulator of ferroptosis. TRIM3 is downregulated in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC), two major types of non-small cell lung cancer (NSCLC). Forced expression of TRIM3 promotes cell death by enhancing the cellular level of ROS and lipid peroxidation. Moreover, our in vivo study determined that TRIM3 overexpression diminishes the tumorigenicity of NSCLC cells, indicating that TRIM3 functions as a tumor suppressor in NSCLC. Mechanistically, TRIM3 directly interacts with SLC7A11/xCT through its NHL domain, leading to SCL7A11 K11-linked ubiquitination at K37, which promotes SLC7A11 proteasome-mediated degradation. Importantly, TRIM3 expression exhibits a negative correlation with SCL7A11 expression in clinical NSCLC samples, and low TRIM3 expression is associated with a worse prognosis. This study reveals that TRIM3 functions as a tumor suppressor that can impede the tumorigenesis of NSCLC by degrading SLC7A11, suggesting a novel therapeutic strategy against NSCLC.
TRIM3通过促进SLC7A11/xCT k11相关的泛素化和降解,促进非小细胞肺癌的铁凋亡。
铁下垂是一种独特的受调节的坏死细胞死亡形式,是由过度的铁依赖性脂质过氧化引起的。然而,人类癌症中驱动铁下垂的潜在机制仍然难以捉摸。在这项研究中,我们发现TRIM3,一种E3泛素蛋白连接酶,是铁死亡的关键调节因子。TRIM3在肺腺癌(LUAD)和肺鳞状细胞癌(LUSC)两种主要的非小细胞肺癌(NSCLC)中下调。强迫表达TRIM3通过提高细胞ROS水平和脂质过氧化促进细胞死亡。此外,我们的体内研究发现TRIM3过表达降低了NSCLC细胞的致瘤性,表明TRIM3在NSCLC中具有抑瘤作用。在机制上,TRIM3通过其NHL结构域直接与SLC7A11/xCT相互作用,导致SCL7A11 k11连接的泛素化K37,从而促进SLC7A11蛋白酶体介导的降解。重要的是,在临床NSCLC样本中,TRIM3表达与SCL7A11表达呈负相关,低TRIM3表达与较差的预后相关。本研究发现TRIM3作为肿瘤抑制因子,可通过降解SLC7A11抑制NSCLC的肿瘤发生,提示一种新的治疗NSCLC的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


