A facile Agrobacterium-mediated transformation method for the model unicellular green algae Chlamydomonas reinhardtii
In Vitro Cellular & Developmental Biology – Plant
Pub Date : 2023-11-13
DOI:10.1007/s11627-023-10389-7
引用次数: 0
Abstract
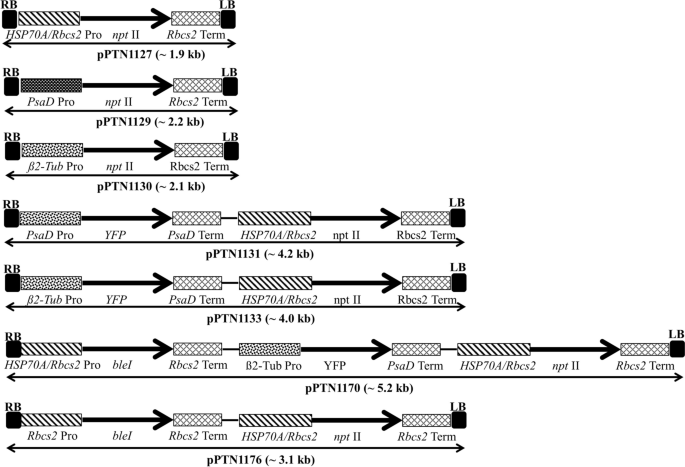
Abstract A reliable and simple Agrobacterium -mediated transformation system for the unicellular green algae model organism Chlamydomonas reinhardtii has been developed. The protocol has been successfully employed with both neomycin phosphotransferase II ( nptII ) and the phleomycin resistance ( bleI ) genes coupled with the selective agents paromomycin and zeocin, respectively. A set of binary vectors were assembled that carry the selectable marker cassettes under control either of the Rbcs2 alone or fused to the HSP270A leader sequence, PsaD, or ß-tubulin2 promoters. The corresponding T-DNA elements also harbored a cassette with a codon-optimized version of yellow fluorescence protein (YFP) under control of the Rbcs2 promoter in which the YFP open reading frame was interrupted with the first intron of Rbcs2 to prevent expression in Agrobacterium tumefaciens . The resultant binary vectors were introduced into A. tumefaciens strain C58C1/pMP90, and the derived transconjugants were used for transformation studies with the walled C. reinhardtii strain CC124. Estimated transformation frequencies ranged from 0.09 to 2.86 colonies per 10 6 cells inoculated. Molecular characterizations on a subset of the transgenic lineages revealed that most of the transgenic events harbored single locus insertions. Moreover, sequencing of captured junction fragments about the T-DNA insertion site showed that minimal disruption of the C. reinhardtii genome occurred. However, the transgenic lineages often harbored truncated T-DNA regions within the non-selectable marker gene cassettes.
农杆菌介导模式单细胞绿藻莱茵衣藻转化的简易方法
摘要建立了一种可靠、简单的农杆菌介导的单细胞绿藻模式生物莱茵衣藻转化体系。该方案已成功地将新霉素磷酸转移酶II (nptII)和白霉素耐药基因(bleI)分别与选择性药物paromomycin和zeocin偶联。在Rbcs2单独或与HSP270A先导序列、PsaD或ß-tubulin2启动子融合的控制下,组装了一组携带可选择标记盒的二进制载体。相应的T-DNA元件还包含一个由Rbcs2启动子控制的密码子优化版本的黄色荧光蛋白(YFP)盒,其中YFP开放阅读框被Rbcs2的第一个内含子打断,以防止在农杆菌中表达。将得到的二元载体导入瘤化假单胞菌C58C1/pMP90中,并将得到的转偶联物与带有壁的莱茵哈德假单胞菌CC124进行转化研究。估计转化频率为0.09至2.86菌落/ 106个接种细胞。对一部分转基因谱系的分子特征分析表明,大多数转基因事件都包含单位点插入。此外,对捕获的T-DNA插入位点的连接片段进行测序表明,莱茵哈特瓢虫基因组发生了最小的破坏。然而,转基因谱系通常在非选择性标记基因盒中含有截断的T-DNA区域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


