Visible light-mediated hydrogen atom transfer and proton transfer for the conversion of (2-vinylaryl)methanol derivatives to aryl aldehydes or aryl ketones†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
In this paper, we report a photochemical strategy for the visible light-mediated efficient conversion of (2-vinylaryl)methanol derivatives to the corresponding aryl aldehydes or aryl ketones in moderate to excellent yields with broad substrate scope under mild conditions. This photochemical process takes place from the generation of the triplet state of olefins and involves 1,5-hydrogen atom transfer, enol tautomerization, and a subsequent proton transfer process. The plausible reaction mechanism has been verified by deuterium labeling and control experiments, kinetic and Stern–Volmer analyses, and DFT calculations.
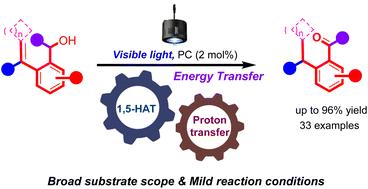
可见光介导的(2-乙烯基)甲醇衍生物转化芳醛或芳酮的氢原子转移和质子转移
开发了一种可见光介导的(2-乙烯芳基)甲醇衍生物高效转化为芳基醛或芳基酮的策略。这一合理的反应机理已被一系列的机理研究所证实。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


