Oxidation of a triple carbo[5]helicene with hypervalent iodine†
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The reactivities of both diastereomers of hexabenzotriphenylene (HBTP), a triple carbo[5]helicene of the formula C42H24, were examined in the presence of phenyliodine diacetate (PIDA) as an oxidizing agent. The D3-symmetric diastereomer afforded two different ring rearranged ketone-containing products embedding a spirofluorene moiety. In contrast, under similar conditions, the C2-symmetric diastereomer afforded predominantly a cyclodehydrogenation product. Altogether, this shows that stereochemistry is a critical factor in the reactivity of non-planar polycyclic aromatic hydrocarbons.
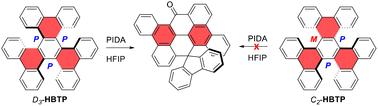
高价碘氧化三碳[5]螺旋烯
立体化学是影响典型三碳[5]螺旋烯六苯并三苯(HBTP)非对映体反应活性的关键因素:d3 -HBTP可以转化为氧化重排产物,但c2 -HBTP不能转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


