Christine M. Swanson, Kelly Krohn, Alexander Wiseman, Micol S. Rothman
{"title":"New Human Leukocyte Antigen (HLA) Antibody Formation and Creatinine Elevation With Abaloparatide in Kidney Transplant Recipient","authors":"Christine M. Swanson, Kelly Krohn, Alexander Wiseman, Micol S. Rothman","doi":"10.1002/jbm4.10814","DOIUrl":null,"url":null,"abstract":"<p>A 39-year-old female with a history of kidney transplant presented to the endocrinology clinic for osteoporosis evaluation after sustaining an ankle fracture from a fall. Her kidney transplant regimen (mycophenolate mofetil 360 mg twice a day, tacrolimus 0.5 mg every morning and 0.5–1 mg every evening, prednisone 5 mg/day) and baseline creatinine (1.0–1.2 mg/dL) had been stable for several years. After an appropriate secondary workup, she was started on abaloparatide 80 μg subcutaneous daily injections for osteoporosis. She had a good initial biochemical response to therapy. However, 5 months after abaloparatide initiation she was found to have a new elevation in serum creatinine (1.17 to 1.69 mg/dL) despite stable serum tacrolimus trough levels, and two new human leukocyte antigen (HLA) antibodies (anti-HLA antibodies detected to Cw7 and DP28). Abaloparatide was stopped due to concern for immunogenicity. There was no evidence of rejection on kidney biopsy and she was restabilized on her transplant regimen with a new baseline creatinine of 1.3–1.6 mg/dL. The patient was subsequently started on teriparatide 20 μg daily subcutaneous injections for 2 years with good biochemical response, significant improvement in bone mineral density, and stable transplant regimen without additional signs of immunogenicity or rejection. This is the first case report to raise concern about immunogenicity with abaloparatide in solid organ transplant recipients. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.</p>","PeriodicalId":14611,"journal":{"name":"JBMR Plus","volume":"7 12","pages":""},"PeriodicalIF":3.4000,"publicationDate":"2023-09-21","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10814","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"JBMR Plus","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10814","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0
Abstract
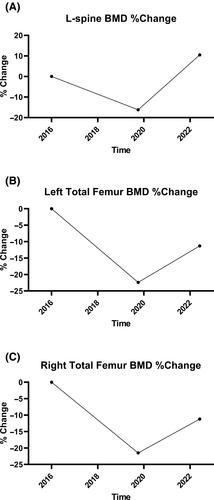
A 39-year-old female with a history of kidney transplant presented to the endocrinology clinic for osteoporosis evaluation after sustaining an ankle fracture from a fall. Her kidney transplant regimen (mycophenolate mofetil 360 mg twice a day, tacrolimus 0.5 mg every morning and 0.5–1 mg every evening, prednisone 5 mg/day) and baseline creatinine (1.0–1.2 mg/dL) had been stable for several years. After an appropriate secondary workup, she was started on abaloparatide 80 μg subcutaneous daily injections for osteoporosis. She had a good initial biochemical response to therapy. However, 5 months after abaloparatide initiation she was found to have a new elevation in serum creatinine (1.17 to 1.69 mg/dL) despite stable serum tacrolimus trough levels, and two new human leukocyte antigen (HLA) antibodies (anti-HLA antibodies detected to Cw7 and DP28). Abaloparatide was stopped due to concern for immunogenicity. There was no evidence of rejection on kidney biopsy and she was restabilized on her transplant regimen with a new baseline creatinine of 1.3–1.6 mg/dL. The patient was subsequently started on teriparatide 20 μg daily subcutaneous injections for 2 years with good biochemical response, significant improvement in bone mineral density, and stable transplant regimen without additional signs of immunogenicity or rejection. This is the first case report to raise concern about immunogenicity with abaloparatide in solid organ transplant recipients. © 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
肾移植受者服用阿巴帕肽后新的人类白细胞抗原 (HLA) 抗体形成和肌酐升高
一名 39 岁的女性患者因摔倒导致踝关节骨折,曾接受过肾移植手术,后到内分泌科门诊进行骨质疏松症评估。她的肾移植治疗方案(霉酚酸酯 360 毫克,每天两次;他克莫司 0.5 毫克,每天早上和晚上各 0.5-1 毫克;泼尼松 5 毫克/天)和基线肌酐(1.0-1.2 毫克/分升)已稳定数年。在进行了适当的辅助检查后,她开始每天皮下注射阿巴拉帕肽 80 μg,以治疗骨质疏松症。治疗初期,她的生化反应良好。然而,在开始使用阿巴帕肽 5 个月后,尽管血清他克莫司谷值水平稳定,但她发现血清肌酐再次升高(1.17 至 1.69 毫克/分升),并出现两种新的人类白细胞抗原(HLA)抗体(检测到 Cw7 和 DP28 抗 HLA 抗体)。由于担心免疫原性,阿巴帕肽被停用。肾活检未发现排斥反应,她的移植治疗方案恢复稳定,新的基线肌酐为 1.3-1.6 mg/dL。随后,患者开始使用特立帕肽 20 μg,每天皮下注射,持续 2 年,生化反应良好,骨矿物质密度明显改善,移植方案稳定,没有出现其他免疫原性或排斥迹象。这是第一例引起人们对阿巴帕肽在实体器官移植受者中的免疫原性关注的病例报告。© 2023 作者。JBMR Plus 由 Wiley Periodicals LLC 代表美国骨矿研究学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


