Direct Reductive Coupling of Nitro Compounds for the Synthesis of Advanced Amines
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Abstract Direct reductive coupling of nitro compounds with C-coupling partners is an atom- and step-economical strategy to access polyfunctional advanced amines. Due to the extremely complex process involved in the reduction of nitro compounds and the high reactivity of N,O-intermediates, few reliable methodologies have been reported for the reductive coupling of nitro compounds since the initial studies. To address this significant challenge, numerous endeavors have been devoted to this important area over the past hundred years. In this short review, we summarize recent advances in this domain and discuss the mechanisms of these appealing reductive coupling transformations. 1 Introduction 2 Reductive Coupling of Nitro Compounds with Organometallic Reagents 3 Reductive Coupling of Nitro Compounds with Arylboronic Acids 4 Reductive Coupling of Nitro Compounds with Alkenes 5 Reductive Coupling of Nitro Compounds with Alkyl/Aryl Halides 6 Reductive Coupling of Nitro Compounds with Alcohols and Their Derivatives 7 Conclusion
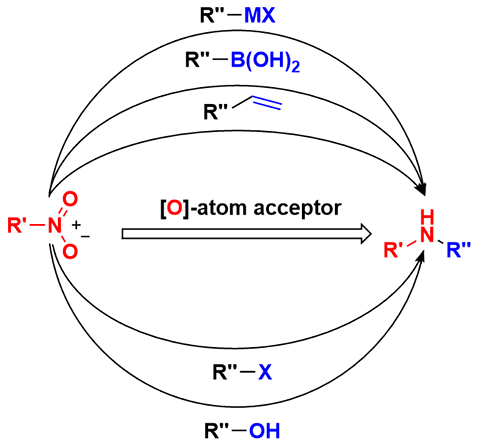
硝基化合物直接还原偶联合成高级胺
硝基化合物与c -偶联伙伴的直接还原偶联是一种原子和步骤经济的策略,以获得多功能高级胺。由于硝基化合物的还原过程极其复杂,且N, o中间体具有较高的反应活性,因此从最初的研究开始,关于硝基化合物的还原偶联的可靠方法报道很少。为了应对这一重大挑战,在过去的一百年里,人们在这一重要领域做出了许多努力。在这篇简短的综述中,我们总结了这一领域的最新进展,并讨论了这些吸引人的还原耦合转换的机制。1导论2硝基化合物与有机金属试剂的还原偶联3硝基化合物与芳基硼酸的还原偶联4硝基化合物与烯烃的还原偶联5硝基化合物与烷基/芳基卤化物的还原偶联6硝基化合物与醇及其衍生物的还原偶联7结论
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


