N-Oxide Route to the Marine Natural Product Cyanogramide D
IF 2.3
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The first synthesis of the marine natural product cyanogramide D is reported. The key step is the acetylation of a β-carboline N-oxide, followed by acetyl migration. Since in this particular case it was not possible to incorporate the styryl side chain by Buchwald coupling, a phenylethanolamine side chain was attached, which was dehydrated with Martin's sulfurane after assembly of the tetracycle. Pentacyclic products were obtained under Appel conditions. The synthesis will facilitate the exploration of the biomimetic oxidative spirocyclization of cyanogramide D to the spirooxindole cyanogramide.
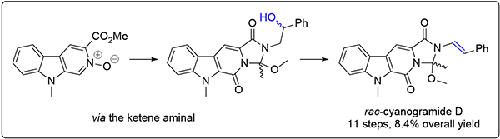
海洋天然产物氰酰胺的n -氧化物途径
报道了海洋天然产物氰酰胺D的首次合成。关键步骤是β-碳碱n -氧化物的乙酰化,然后是乙酰化迁移。由于在这种特殊情况下,不可能通过布赫瓦尔德偶联结合苯乙烯侧链,因此连接了苯乙醇胺侧链,在四环组装后用马丁硫烷脱水。在Appel条件下得到了五环产物。该合成将有助于探索氰酰胺D的仿生氧化螺旋环化制螺旋吲哚型氰酰胺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


