Precision tuning of highly efficient Pt-based ternary alloys on nitrogen-doped multi-wall carbon nanotubes for methanol oxidation reaction
Abstract
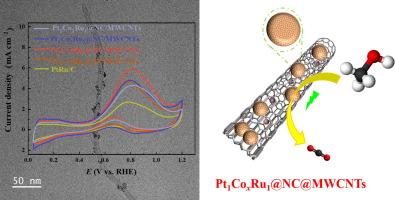
The electrochemical methanol oxidation is a crucial reaction in the conversion of renewable energy. To enable the widespread adoption of direct methanol fuel cells (DMFCs), it is essential to create and engineer catalysts that are both highly effective and robust for conducting the methanol oxidation reaction (MOR). In this work, trimetallic PtCoRu electrocatalysts on nitrogen-doped carbon and multi-wall carbon nanotubes (PtCoRu@NC/MWCNTs) were prepared through a two-pot synthetic strategy. The acceleration of CO oxidation to CO2 and the blocking of CO reduction on adjacent Pt active sites were attributed to the crucial role played by cobalt atoms in the as-prepared electrocatalysts. The precise control of Co atoms loading was achieved through precursor stoichiometry. Various physicochemical techniques were employed to analyze the morphology, element composition, and electronic state of the catalyst. Electrochemical investigations and theoretical calculations confirmed that the Pt1Co3Ru1@NC/MWCNTs exhibit excellent electrocatalytic performance and durability for the process of MOR. The enhanced MOR activity can be attributed to the synergistic effect between the multiple elements resulting from precisely controlled Co loading content on surface of the electrocatalyst, which facilitates efficient charge transfer. This interaction between the multiple components also modifies the electronic structures of active sites, thereby promoting the conversion of intermediates and accelerating the MOR process. Thus, achieving precise control over Co loading in PtCoRu@NC/MWCNTs would enable the development of high-performance catalysts for DMFCs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


