Personalized treatment in localized pancreatic cancer
引用次数: 0
Abstract
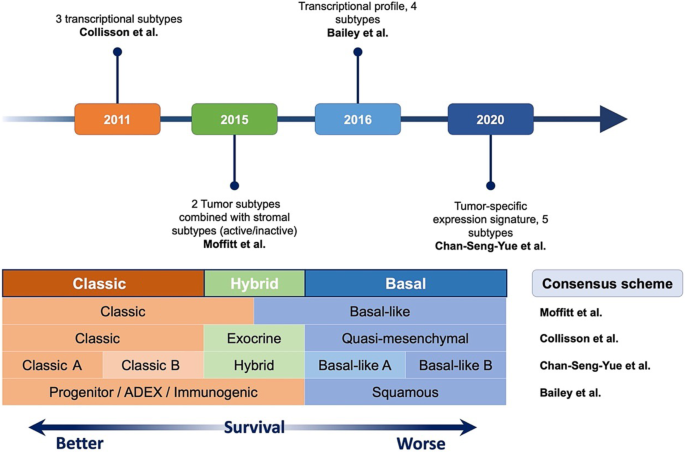
Summary The treatment elements used for pancreatic ductal adenocarcinoma (PDAC) include surgical resection, systemic cytotoxic agents, and targeted drugs. For second- and third-line therapies in PDAC, approximately 15% of patients have actionable mutations although only 2.5% receive matched targeted treatment but with a significant improvement in survival of around 16 months. For the majority of PDAC patients the current most effective strategy is surgical resection of the primary tumor and systemic combination chemotherapy. The chemotherapy regimens and the order of delivery relative to the resection reference point have been based to a large extent on randomized trials using a newly developed empirical staging (Em) system. Although the reductionist TNM based AJCC and UICC systems work well for pathology staging, they are less accurate and less manageable for treatment decision-making. This Em system defines locally resectable (EmR), borderline resectable (EmBR), and unresectable (EmUR) stages, plus the emerging entity of oligometastatic disease (EmOm). For EmR patients, 6 months of adjuvant chemotherapy achieves 5‑year survival rates of 30–50%. In EmBR short-course (2 months) neoadjuvant plus 6‑month adjuvant chemotherapy increases 12-month survival rates to around 77%, compared to 40% for upfront surgery, despite resection rates of 64–85% and 75%, respectively. Longer-course (4 months) neoadjuvant chemotherapy has also been shown to achieve an 18-month overall survival of 67%. In EmUR, induction therapy (3–6 months) may result in resections rates of 20–60% with significantly improved survival rates compared to no resection. For all stages including the polymetastatic (EmPm) setting, patients with good performance status receive combination chemotherapies based on either oxaliplatin (FOLFIRINOX or NALIRIFOX) or gemcitabine (GEM-CAP, or Gem-NabP). Molecular subtypes (Moffitt, Collisson, Bailey, and Cheng-Sen-Yue) are shown to be associated with treatment responses. Transcriptomic signatures have also been developed as classifiers for determining either oxaliplatin- or gemcitabine-based therapies (PurIST, Tiriac, GemPred+, and ESPAC) and are being evaluated in various studies. Most notably the ESPAC transcriptomic signature is being used as the treatment classifier in the experimental arms of the randomized ESPAC6 adjuvant trial in EmR patients and the ESPAC7 induction therapy trial in EmUR patients. Genomic and transcriptomic profiling at baseline and over time is an integral part of ESPAC6/7 to deepen our understanding of tumor plasticity during the course of therapy, identifying the intrinsic (persister cell) and acquired (genetic) tumor plasticity evolving over time and in reaction to different therapies in order to enable a scientific approach to overcoming clonal-resistance clades.
局部胰腺癌的个体化治疗
胰腺导管腺癌(PDAC)的治疗方法包括手术切除、全身细胞毒性药物和靶向药物。对于PDAC的二线和三线治疗,大约15%的患者有可操作的突变,尽管只有2.5%的患者接受了匹配的靶向治疗,但生存率显著提高,约为16个月。对于大多数PDAC患者,目前最有效的策略是手术切除原发肿瘤和全身联合化疗。化疗方案和相对于切除参考点的递送顺序在很大程度上是基于使用新开发的经验分期(Em)系统的随机试验。虽然基于还原论TNM的AJCC和UICC系统在病理分期方面效果很好,但它们在治疗决策方面不太准确,也不太容易管理。该Em系统定义了局部可切除(EmR)、边缘可切除(EmBR)和不可切除(EmUR)阶段,以及新出现的少转移性疾病(EmOm)。对于EmR患者,6个月的辅助化疗可实现30-50%的5年生存率。在EmBR短期疗程(2个月)中,新辅助+ 6个月辅助化疗将12个月生存率提高至77%左右,而前期手术的生存率为40%,尽管切除率分别为64-85%和75%。较长疗程(4个月)的新辅助化疗也显示可达到67%的18个月总生存率。在EmUR中,诱导治疗(3-6个月)可能导致20-60%的切除率,与不切除相比,生存率显著提高。对于包括多转移性(EmPm)的所有阶段,表现良好的患者接受基于奥沙利铂(FOLFIRINOX或NALIRIFOX)或吉西他滨(GEM-CAP或Gem-NabP)的联合化疗。分子亚型(Moffitt, Collisson, Bailey和Cheng-Sen-Yue)被证明与治疗反应有关。转录组特征也被开发为确定奥沙利铂或吉西他滨为基础的治疗(PurIST, Tiriac, GemPred+和ESPAC)的分类器,并正在各种研究中进行评估。最值得注意的是,在EmR患者的随机ESPAC6辅助试验和EmUR患者的ESPAC7诱导治疗试验的实验组中,ESPAC转录组特征被用作治疗分类器。基线和随时间变化的基因组和转录组分析是ESPAC6/7的一个组成部分,旨在加深我们对治疗过程中肿瘤可塑性的理解,识别固有的(持续细胞)和获得的(遗传)肿瘤可塑性随着时间的推移和对不同治疗的反应,从而使科学方法能够克服克隆抗性进化枝。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


