Thin layer chromatography‒spectrodensitometric determination of a three-component mixture of propyphenazone, caffeine, ergotamine tartrate, and two of their impurities with application to tablets, spiked human plasma, and green profile assessment
JPC – Journal of Planar Chromatography – Modern TLC
Pub Date : 2023-09-25
DOI:10.1007/s00764-023-00248-x
引用次数: 0
Abstract
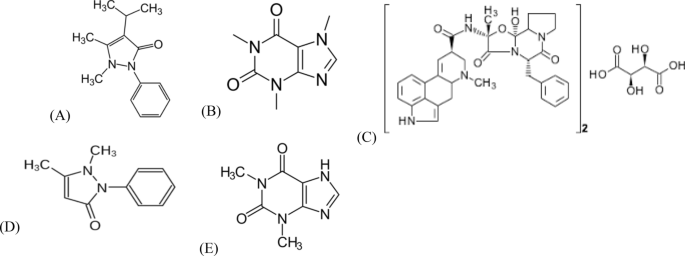
Abstract A novel, green, and cost-effective thin-layer chromatography (TLC)‒spectrodensitometric method was designed and validated for the simultaneous determination of a five-component mixture. The analyzed mixture is composed of three active ingredients: propyphenazone (PRO), caffeine (CAF), and ergotamine tartrate (ERG), along with two official impurities which are PRO impurity: phenazone (PHN) and CAF impurity: theophylline (THEO). The suggested method was used for the quantitation of the three coformulated active ingredients in their marketed tablet and in human plasma. The studied compounds were separated on TLC silica gel 60F 254 plates using a mobile phase consisting of methanol–ethyl acetate–glacial acetic acid (1:9:0.1, V/V ) with diprophylline (DPP) as internal standard. Densitometric scanning was carried out at 210.0 nm. Method validation was assessed according to the International Council for Harmonisation (ICH) guidelines. The greenness profile for the proposed method was evaluated using the National Environmental Method Index (NEMI), analytical eco-scale, and Green Analytical Procedure Index (GAPI) tools. The proposed method offers the advantages of being simple, rapid, economic, and ecofriendly. It is a successful choice for the routine analysis of the studied drugs in pharmaceutical and biological samples.
薄层色谱-光谱密度法测定丙苯那酮、咖啡因、酒石酸麦角胺及其两种杂质的三组分混合物,并应用于片剂、加标人血浆和绿色谱评估
摘要:设计了一种新型、绿色、经济的薄层色谱-光谱密度法,用于同时测定五组分混合物。所分析的混合物由三种有效成分组成:丙基非那酮(PRO)、咖啡因(CAF)和酒石酸麦角胺(ERG),以及两种官方杂质,即PRO杂质:非那酮(PHN)和CAF杂质:茶碱(THEO)。所建议的方法用于三种共配制的有效成分在其上市片剂和人血浆中的定量。采用甲醇-乙酸乙酯-冰醋酸(1:9:0.1,V/V)为流动相,以二苯甲酸(DPP)为内标,在薄层色谱60f254硅胶板上分离所得化合物。在210.0 nm处进行密度扫描。方法验证根据国际协调理事会(ICH)指南进行评估。采用国家环境方法指数(NEMI)、分析生态尺度和绿色分析程序指数(GAPI)工具对所提出方法的绿色概况进行了评估。该方法具有简单、快速、经济、环保等优点。它是制药和生物样品中所研究药物的常规分析的成功选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


