Structure, Frontier Molecular Orbitals, MEP, Charge Analysis, and NLO Study of 2,4-, 2,5-, and 2,6-Dimethylanilines Using DFT
IF 2.4
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
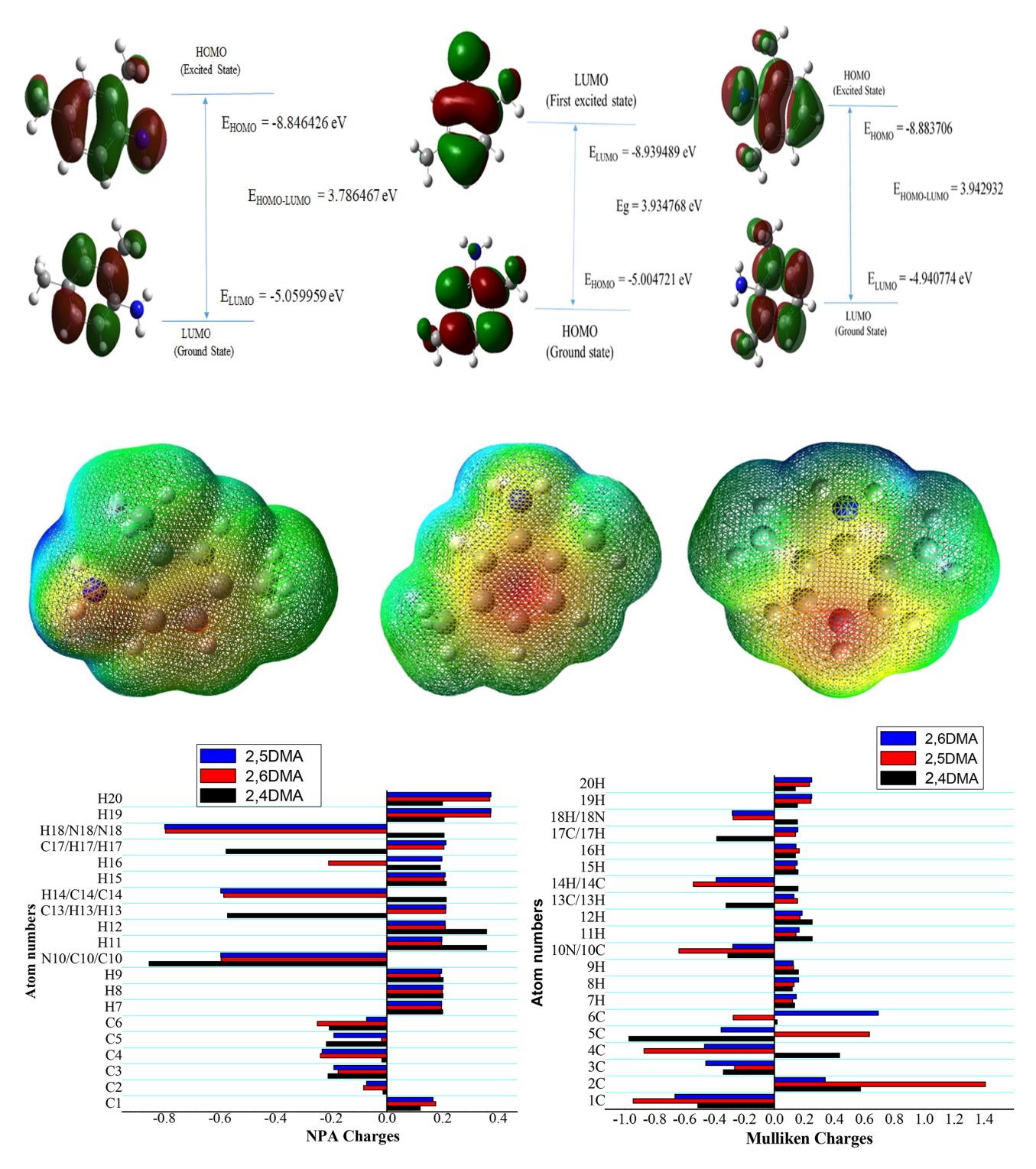
This study includes the evaluation of most stable structure, frontier molecular orbitals, molecular electrostatic potential (MEP), and nonlinear optical (NLO) parameters of 2,4-dimethylaniline (2,4DMA), 2,5-dimethylaniline (2,5DMA), and 2,6-dimethylaniline (2,6DMA) using density functional theory (DFT) employing B3LYP functional with 6-311++G(d,p) basis set. HOMO-LUMO energies, band gap energies, and global reactivity descriptors were obtained from the optimized structures. The band gap energy was determined as 3.7865, 3.9348, and 3.9443 eV for 2,4DMA, 25DMA, and 2,6DMA, respectively. The reactive sites of the molecules were obtained from the molecular electrostatic potential energy. The NLO parameters such as dipole moment, polarizability, and hyperpolarizability have been investigated for the title molecules. The investigated dipole moment and first-order hyperpolarizability values are higher than the prototypical value of urea and show good NLO behavior. The NMR chemical shifts, electronic absorption spectra, and charge distribution of atoms were calculated theoretically. Furthermore, thermodynamic and rotational constants were also calculated.
利用 DFT 对 2,4-、2,5- 和 2,6-Dimethylanilines 进行结构、前沿分子轨道、MEP、电荷分析和 NLO 研究
本研究采用密度泛函理论 (DFT),使用 6-311++G(d,p) 基集的 B3LYP 函数,对 2,4 二甲基苯胺 (2,4DMA)、2,5 二甲基苯胺 (2,5DMA) 和 2,6 二甲基苯胺 (2,6DMA) 的最稳定结构、前沿分子轨道、分子静电位 (MEP) 和非线性光学 (NLO) 参数进行了评估。从优化结构中获得了 HOMO-LUMO 能、带隙能和全局反应性描述符。经测定,2,4DMA、25DMA 和 2,6DMA 的带隙能分别为 3.7865、3.9348 和 3.9443 eV。根据分子静电势能得出了分子的反应位点。对标题分子的偶极矩、极化率和超极化率等 NLO 参数进行了研究。所研究的偶极矩和一阶超极化率值均高于脲的原型值,显示出良好的 NLO 行为。核磁共振化学位移、电子吸收光谱和原子的电荷分布都是通过理论计算得出的。此外,还计算了热力学常数和旋转常数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polycyclic Aromatic Compounds
化学-有机化学
CiteScore
3.70
自引率
20.80%
发文量
412
审稿时长
3 months
期刊介绍:
The purpose of Polycyclic Aromatic Compounds is to provide an international and interdisciplinary forum for all aspects of research related to polycyclic aromatic compounds (PAC). Topics range from fundamental research in chemistry (including synthetic and theoretical chemistry) and physics (including astrophysics), as well as thermodynamics, spectroscopy, analytical methods, and biology to applied studies in environmental science, biochemistry, toxicology, and industry. Polycyclic Aromatic Compounds has an outstanding Editorial Board and offers a rapid and efficient peer review process, as well as a flexible open access policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


