Efficacy and safety of zilucoplan in Japanese patients with generalized myasthenia gravis: A subgroup analysis of the phase III randomized RAISE study
Abstract
Objectives
RAISE (NCT04115293) was a randomized, multicenter, double-blind, placebo-controlled phase III study of zilucoplan, a macrocyclic peptide and complement component 5 inhibitor with a dual mechanism of action, in patients with acetylcholine receptor autoantibody-positive generalized myasthenia gravis (MG). RAISE showed clinically meaningful and statistically significant improvements in MG-specific outcomes in the overall population. Here, we assess efficacy and safety of zilucoplan in patients with generalized myasthenia gravis in the Japanese patients enrolled in RAISE.
Methods
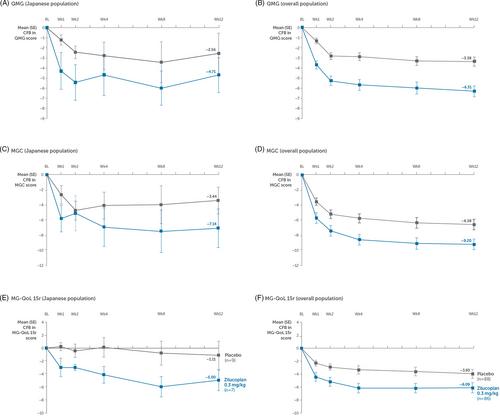
Adults with acetylcholine receptor autoantibody-positive generalized MG (MGFA disease class II–IV) were randomized 1:1 to daily self-administered subcutaneous zilucoplan 0.3 mg/kg or placebo injections for 12 weeks. The primary efficacy endpoint was change from baseline to week 12 in the MG Activities of Daily Living score. Safety was assessed by the incidence of treatment-emergent adverse events. Efficacy and safety outcomes of a Japanese subgroup were prespecified.
Results
Overall, 16 Japanese patients were randomized to zilucoplan 0.3 mg/kg (n = 7) or placebo (n = 9). There was a clinically meaningful improvement in the MG Activities of Daily Living score at week 12 for zilucoplan versus placebo in the Japanese population, with least squares mean difference of −4.26 (95% confidence interval −7.40, −1.12), which was comparable with the overall population. The incidence of treatment-emergent adverse events was similar in both treatment arms, with 57.1% and 55.6% of patients in the zilucoplan and placebo groups, respectively, experiencing treatment-emergent adverse events.
Conclusions
In a subgroup of Japanese patients, zilucoplan showed clinically meaningful improvement in MG-specific outcomes with a favorable safety profile, consistent with the overall RAISE population.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


