Electronic structure and spin state regulation of vanadium nitride via a sulfur doping strategy toward flexible zinc-air batteries
Abstract
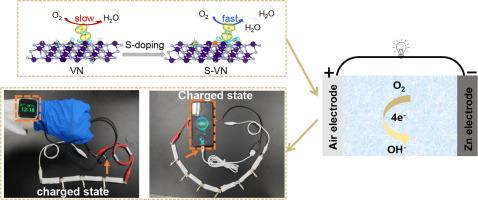
Owing to the distinctive structural characteristics, vanadium nitride (VN) is highly regarded as a catalyst for oxygen reduction reaction (ORR) in zinc-air batteries (ZABs). However, VN exhibits limited intrinsic ORR activity due to the weak adsorption ability to O-containing species. Here, the S-doped VN anchored on N, S-doped multi-dimensional carbon (S-VN/Co/NS-MC) was constructed using the solvothermal and in-situ doping methods. Incorporating sulfur atoms into VN species alters the electron spin state of vanadium in the S-VN/Co/NS-MC for regulating the adsorption energy of vanadium sites to oxygen molecules. The introduced sulfur atoms polarize the V 3dz2 electrons, shifting spin-down electrons closer to the Fermi level in the S-VN/Co/NS-MC. Consequently, the introduction of sulfur atoms into VN species enhances the adsorption energy of vanadium sites for oxygen molecules. The *OOH dissociation transitions from being unspontaneous on the VN surface to a spontaneous state on the S-doped VN surface. Then, the ORR barrier on the S-VN/Co/NS-MC surface is reduced. The S-VN/Co/NS-MC demonstrates a higher half-wave potential and limiting current density compared to the VN/Co/N-MC. The S-VN/Co/NS-MC-based liquid ZABs display a power density of 195.7 mW cm−2, a specific capacity of 815.7 mA h g−1, and a cycling stability exceeding 250 h. The S-VN/Co/NS-MC-based flexible ZABs are successfully employed to charge both a smart watch and a mobile phone. This approach holds promise for advancing the commercial utilization of VN-based catalysts in ZABs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


