Synthetic Access to 1,3-Butadiynes via Electro-redox Cuprous Catalyzed Dehydrogenative Csp-Csp Homocoupling of Terminal Acetylenes
IF 2.2
4区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we have disclosed the oxidative homocoupling of terminal alkynes under electrochemically generated cuprous catalysis. The scope of this protocol is established by preparing an array of structurally and electronically different 1,3-butadiyne derivatives. Good synthetic yields, functional group tolerance, oxidant-free conditions and no cross-selectivity are some of the intrinsic advantages of this methodology. The developed chemistry features the electro-redox formation of copper-acetylide, an intermediate appropriate for the Csp-Csp coupling step. The chemical state of copper in the acetylide intermediate was found be Cu(I) as confirmed by click-trapping experiments, CV, EPR and XPS. Competition reaction to determine the reactivity of electronically dissimilar acetylenes revealed that the product ratio is rather dependent on the electronic nature of alkynyl substituents. To highlight the synthetic value of the products, selected diynes were subjected to chemical diversification.
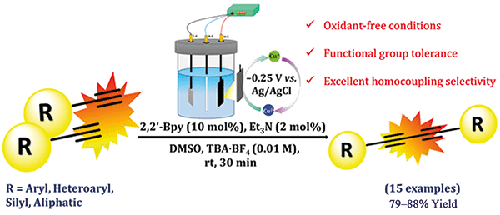
电氧化还原亚铜催化末端乙炔脱氢Csp-Csp均偶联制备1,3-丁二炔
在此,我们公开了在电化学生成的亚铜催化下末端炔的氧化均偶联。本议定书的范围是通过制备一系列结构上和电子上不同的1,3-丁二炔衍生物来确定的。该方法具有合成收率高、官能团耐受性好、无氧化条件和无交叉选择性等优点。发展的化学特征是电氧化还原形成铜-乙酰酯,一种适合于Csp-Csp耦合步骤的中间体。通过点击捕获实验、CV、EPR和XPS证实,乙酰基中间体中铜的化学态为Cu(I)。用竞争反应测定电子不相似乙炔的反应活性,结果表明,产物比很大程度上取决于炔基取代基的电子性质。为了突出产品的合成价值,选择的diynes进行了化学多样化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthesis-Stuttgart
化学-有机化学
CiteScore
4.50
自引率
7.70%
发文量
435
审稿时长
1 months
期刊介绍:
SYNTHESIS is an international full-paper journal devoted to the advancement of the science of chemical synthesis. It covers all fields of organic chemistry involving synthesis, including catalysis, organometallic, medicinal, biological, and photochemistry, but also related disciplines. SYNTHESIS provides dependable research results with detailed and reliable experimental procedures and full characterization of all important new products as well as scientific primary data.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


