Rhodamine B sequestration using acid-precipitated and microwave-treated softwood lignin: Comparative isotherm, kinetics and thermodynamic studies
引用次数: 0
Abstract
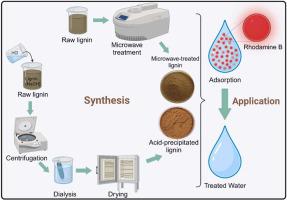
Sustainable development goals have emphasized the need to focus on water treatment to mitigate the ever-rising demands for clean water. High costs and energy requirements have been the Achilles’ heel of most treatment technologies. Herein, we develop cheap lignin-derived sorbents prepared by microwave (MW) and acid precipitation (AP) treatments of raw lignin (RL) for Rhodamine-B (RhB) adsorption in aqueous solution. Our findings evidence micro- and mesoporous structures, with irregular grain size. X-ray diffraction revealed an amorphous material, while Fourier-transform infrared analysis points to the presence of carboxyl, hydroxyl and sulfite functional groups, which may facilitate the adsorption of the dye. Adsorption isotherm and kinetic data describe complex and pore-driven interactions, based on the Sips, D-R and intraparticle diffusion models, considering the sum of squared error values obtained from nonlinear regression analysis. Adsorption efficiency of ∼97% is obtainable at optimal conditions for treated lignin (3 g/L sorbent dose, 720 min contact time and under acidic pH conditions). Thermodynamic studies revealed that RL-RhB and MW-RhB are driven by physisorption considering ∆H° values, while stronger interactions occurred for AP-RhB, considering the high enthalpy (∼49 kJ/mol) and adsorption capacity (∼2766 mg/g). Van der Waals attraction, π-π stacking, and pore-filling mechanisms are among the several interactions that may occur between RhB and the sorbents. Our findings offer a way to valorize lignin as an alternative pathway to economically viable and sustainable water purification.
酸沉淀和微波处理软木木质素的罗丹明B封存:比较等温线、动力学和热力学研究
可持续发展目标强调需要把重点放在水处理上,以减轻对清洁水日益增长的需求。高成本和能源需求一直是大多数处理技术的致命弱点。在此,我们开发了廉价的木质素源吸附剂,通过微波(MW)和酸沉淀(AP)处理原料木质素(RL),制备用于吸附罗丹明- b (RhB)的水溶液。我们的发现证明了微孔和介孔结构,具有不规则的粒度。x射线衍射显示为无定形物质,而傅里叶变换红外分析指出存在羧基,羟基和亚硫酸盐官能团,这可能有助于染料的吸附。基于Sips、D-R和颗粒内扩散模型,考虑非线性回归分析得到的误差平方和,吸附等温线和动力学数据描述了复杂的孔隙驱动相互作用。在处理木质素的最佳条件下(3 g/L吸附剂剂量、720 min接触时间和酸性pH条件下),吸附效率可达97%。热力学研究表明,考虑到∆H°值,RL-RhB和MW-RhB受物理吸附驱动,而AP-RhB考虑到高焓(~ 49 kJ/mol)和高吸附量(~ 2766 mg/g),相互作用更强。范德华吸引、π-π堆积和孔隙填充机制是RhB和吸附剂之间可能发生的几种相互作用。我们的研究结果为木质素作为经济上可行和可持续的水净化的替代途径提供了一种方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


